1. INTRODUCTION
Biodiversity for Food and Agriculture is a major contributor to the resilience of crops, livestock, forests, plant, animal, forestry, fisheries, and aquaculture production systems to respond to environmental change and uncertainty [1]. Conserving the traditional varieties, or agrobiodiversity, is important as a living germplasm bank for the future challenges of agriculture, including climate change [2].
Burkina Faso’s economy is predominantly based on agricultural sectors which employ, along with livestock sectors, 85% of the working population, provide 33% of the gross domestic product, and contribute to 80% of export earnings [3]. From 9 million hectares of land suitable for agriculture, only 3.5 million hectares, representing 39%, are cultivated annually and food grains (sorghum, millet, corn, and rice) alone account for more than 80% of these areas [3]. In 2021, national cereal production was estimated at 4,661,140 tons [4].
Sorghum (Sorghum bicolor [L.] Moench) is the fifth most important cereal in the world [5] and the third one in Africa. Sorghum, along with millet, is the leading cereals in the Sahel, where they cover 50–70% of the cultivable area in the West Africa Sahelian strip [6]. Since 2020, sorghum has been ranked as the second cereal after maize in Burkina Faso with an estimated production of 1,840,000 tons [4,5]. The United States of America remain the biggest producer with 11.38 million tons in 2021, representing 18.18% of total world production, in which volume is estimated at 62.33 million tons [7]. Burkina Faso ranks tenth in the world and fourth in Africa after Nigeria, Ethiopia, and Sudan.
Sorghum generally has a wide range of genetic variability [5]. During the raining season in 2019–2020, Burkina Faso produced 1,425,103 tons of white sorghum and 414,467 tons of red sorghum [6]. These national statistics do not include all available sorghum genetic resources. In addition to grain sorghum, other types of sorghum with multiple potentialities are also exploited. These include sweet sorghums, among which is the sweet stalk sorghum. Sweet stalk sorghum is highly valued in the industry of some countries such as Brazil, India, and the Philippines. The juice yield ranges from 15,200 to 71,100 L/ha depending on varieties and growing conditions [8]. It is used in the production of syrup and biofuels, particularly bioethanol [9,10] with 1,900 L/ha in syrup and 8,000 L/ha in ethanol average yields [11]. The liquid distillate, left after extraction of ethanol from sweet sorghum juice, called vinasse or still age, is used as a fertilizer in agricultural fields [12].
In Burkina Faso, since 2008, many investigations have been undertaken by University of Ouagadougou (current University Joseph Ki-Zerbo) for the sweet stalk sorghum potential. These efforts focused on the characterization of its genetic diversity and the Brix content in the stalk [13-15], the evaluation of the photoperiod sensitivity of genotypes [13], and the genetic relationships between this sorghum and other types of sorghum grown [14-16]. All of these studies have shown significant genetic diversity within sweet stalk sorghum, high sensitivity to photoperiod, and close genetic proximity among grain sorghum and sweet grain sorghum. Seeking additional genetic variability and maximizing the exploitation of existing genetic variability for breeding can facilitate the development of high-yielding and high-performing genotypes [17]. The existence of variability in the population is not sufficient for improving appropriate traits unless genetic variability is well characterized [18]. Analysis of trait variability and the association of a particular trait with others contributing to crop performance is of great importance in a successful breeding scheme [19]. The knowledge of genetic variability and the relationship between traits of interest provides options from which selections can be made for improvement and possible hybridization [18].
The identification of performant genotypes with desired traits and their subsequent use in breeding programs, using appropriate selection criteria, is essential for the success of breeding programs, especially under environmental stress conditions [20]. It is also well established that progress in crop improvement depends not only on the degree of variability of the desired trait in the source material but also on the level of heritability of the desired trait [21]. The knowledge of heritability helps to determine the breeding methods which might be appropriate for plant improvement [22].
Up to now, heritability of agro-morphological traits in sweet stalk sorghum cultivars produced in Burkina Faso remains poorly known. Phenotypic selection based on traits with high broad heritability, coupled with high genetic advance, is most effective for developing desired sorghum genotypes [23]. The direct selection for yield is not effective. It is better to analyze the structure of yield through its components [23]. Genetic improvement of sorghum yield depends on the quality and extent of genetic variability, heritability, and the expected genetic advance in the population as well as the nature of the association between yield and its components [24]. Correlation coefficients help in deciding the direction of selection and the number of traits to be examined for yield improvement [18]. This allows simultaneous selection for many yield-associated traits [18]. This study aims to assess the genetic variability of several sweet stalk sorghum genotypes grown in Burkina Faso, to determine the links between agro-physiological traits and Brix, and to estimate the Shannon-Weaver diversity index of qualitative traits and genetic parameters such as broad sense heritability and expected genetic advance as percent of quantitative traits.
2. MATERIALS AND METHODS
2.1. Plant Material
The plant material was composed of 29 sweet stalk sorghum genotypes from the germplasm of the “Laboratoire Biosciences” of the “Université Joseph Ki-Zerbo.” These genotypes were derived from three cycles of self-pollination of local accessions. These accessions were previously collected in six provinces of Burkina Faso [Table 1].
Table 1: List of 29 sweet stalk sorghum genotypes.
| No. | Area of collection | Lines code | Number of genotypes |
|---|---|---|---|
| 1 | Bam | BBO5, BKB5, BKO2, BSA5, BZI3 | 05 |
| 2 | Gnagna | GBI1, GBI3, GBO2, GBO4, GBO6, GBO8, GBO9 | 07 |
| 3 | Komondjairi | KBA1, KBA2, KBA5, KBA9, KBA10, KGA1, KGA2, KGA6, KGA7, KGA8 | 10 |
| 4 | Lorum | LTI5 | 01 |
| 5 | Namentenga | NBO1, NBO4 | 02 |
| 6 | Soum | SAR7, SDJ2, SPO1, SPO3 | 04 |
2.2. Experimental Site
The trial was set up in July 2019 at the “Institut du Développement Rural” experimental station located in Gampèla at 1° 21’ 9.6’’ west longitude and 12° 24’ 29’’ north latitude. The study site is located in the north Sudanian climatic area, characterized by a short rainy season from June to October and a long dry one from November to May. The soils of the site are very heterogeneous, and deep, with low physiochemical fertility and a predominantly sandy-loam texture [25]. During the experimentation year, the cumulative rainfall was 852.7 mm over 7 months. Rainfall peaked in July (321.3 mm) and, then, decreased in September (212 mm) and stopped in mid-October.
2.3. Experimental Set-up and Cultivation Techniques
The planting areas were plowed and leveled by a tractor and the seedlings were sown on 23 July 2019 in a randomized complete block design with three replications. The replicates were separated from each other by a 2 m row and, in each replicate, each sweet stalk sorghum genotype was sown in a line of 5.2 m long in 14 planting holes. The spacing between the lines was 0.8 m and 0.4 between planting holes. Three weeks after sowing, seedlings were reduced to one plant per hole. Weeding followed by fertilizer application (NPK 15- 15-15) at a rate of 100 kg/ha was done. To reduce weed competition and allow better aeration of the soil, a final weeding was carried out 7 weeks after sowing. Mounding followed by urea application at the rate of 50 kg/ha was carried out at the heading stage to protect the plants from lodging and to preserve soil moisture.
2.4. Data Collection
Eighteen traits including four qualitative traits, 13 agro-physiological traits, and Brix were determined. The four qualitative traits observed were panicle exsertion, panicle shape, grain color, and grain coverage. The 13 agro-physiological traits collected were plant growth, plant cycle, and grain yield. The following plant growth traits, number of vegetative tillers (NVT), plant height (PHT), stem diameter (SDI), internodes number (NIN) and internodes length (INL), third leaf (under panicle) length (LEL), third leaf (under panicle) width (LEW), and peduncle length (PLE) were recorded. Trait related to the life cycle was date (NDF), and the yield traits included the number of productive tillers (NPT), panicle length (PAL), panicle width (PAW), and panicle weight (WPA). Brix was measured at the hard grain stage on three internodes using an ATAGO portable digital refractometer.
2.5. Statistical Analysis
Microsoft Excel 2016 spreadsheet and Xlstat pro version 7.1 software were used for the data analysis. Histograms creation, frequencies, and Shannon-Weaver diversity index (H’) of the qualitative characters were carried out with the Microsoft Excel 2016 spreadsheet program. The Shannon-Weaver diversity index (H’) was determined according to the formula 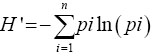 [14,26] where pi is the proportion of accessions in the ith class of an n-class character and η is the number of phenotypic classes of traits. Each H’ value was divided by its maximum value (ln η) and normalized to keep the values between 0 and 1.
[14,26] where pi is the proportion of accessions in the ith class of an n-class character and η is the number of phenotypic classes of traits. Each H’ value was divided by its maximum value (ln η) and normalized to keep the values between 0 and 1.
The quantitative data were subjected to a one-way analysis of variance with Genstat v4. 3 software. The components of the analysis of variance were, then, used to estimate the different genetic parameters of the quantitative traits. Genotypic (δ2g) and phenotypic (δ2p) variances, genotypic (GCV) and phenotypic (PCV) coefficients of variation, broad sense heritability (H2), expected genetic advance (GA), and expected genetic advance as percent mean (GAM) were calculated according to the formulae used in the previous studies [27,28]. Statistica version 6 software was used for Pearson’s correlation test to determine the relationship between Brix and quantitative agro-physiological traits.
3. RESULTS
3.1. Phenotypic Variation in Qualitative Morpho-Agronomic Traits
The results of the analysis of variation of the qualitative traits showed a higher variability of exsertion [Figure 1a], grain coverage [Figure 1b], and grain color [Figure 1c]. Grain shape [Figure 1d] revealed only two types. The Grain showed four different colors ranging from dark red [Figure 2a], light red [Figure 2b], pink [Figure 2c] to gray [Figure 2d], and four level of coverage by the glumes (25%, 50%, 75%, and 100%). Genotypes with dark red grains with <75% glume coverage were the most predominant. In addition, most of the genotypes had positive exsertion (86.21%), while a small proportion (13.79%) had negative or no exsertion. As for grain shape, a very small proportion of the genotypes produced elliptical-shaped grains [Figure 3a], while most of them produced asymmetrically shaped grains [Figure 3b].
 | Figure 1: Frequencies distribution of different modalities of the panicle exsertion (a) grain coverage (b), grain color (c), and grain shape (d) of 29 sweet stalk sorghum genotypes. Bars represent standard error. [Click here to view] |
 | Figure 2: Different grain colors of sweet stalk sorghum genotypes: Dark red (a), light red (b), pink grain (c), and gray grain (d). [Click here to view] |
 | Figure 3: Different grain shapes of sweet stalk sorghum genotypes: Elliptical (a) and asymmetrical (b). [Click here to view] |
3.2. Variation in Agro-Physiological Traits and Brix
All the variables significantly discriminated the sweet stalk sorghum genotypes except for SDI for which no significant difference (F= 1.28; pr. = 0.22) was observed between the 29 lines evaluated [Table 2]. Coefficients of variation of traits were generally low (<30%) with the exception of the tillering-related traits (NVT and NPT) which expressed high values (>40%). Most agronomical and physiological traits expressed relatively high coefficients of determination (R2) (>50%). On the other hand, five traits, Brix (R2 = 45.20%), leaf length (R2 = 40.70%), main panicle weight (R2 = 25.50%), and tillering-related traits (NPV and NPT) had average coefficients of determination between 20% and 50%. Only SDI had a very low R2 value (8.20%). With flowering date varying from 66 days to 82 days, the sweet stalk sorghum lines produced an average of 7.83 vegetative tillers including 3.23 productive tillers and a juice with a Brix value ranging from 12.8% to 24.17%. The greatest environmental (MSE) and genotypic (MSG) variances were observed in PHT with respective values of 333.70 and 4410.80.
Table 2: Results of analysis of variance of the 13 quantitative agro-physiological traits and the Brix of sweet stalk sorghum lines.
| Traits | Min. | Max. | Mean | CV (%)° | F | Pr. | R2 (%) | MSG | MSE |
|---|---|---|---|---|---|---|---|---|---|
| NVT | 0.00 | 11.00 | 4.83 | 47.41 | 2.26 | 0.04 | 29.10 | 8.40 | 3.71 |
| NPT | 0.00 | 6.00 | 3.23 | 46.43 | 1.86 | 0.02 | 21.80 | 3.26 | 1.76 |
| LEL ‘cm) | 52.67 | 80.83 | 65.92 | 8.97 | 3.11 | 0.00 | 40.70 | 64.36 | 19.04 |
| LEW (cm) | 3.30 | 10.17 | 6.16 | 22.17 | 7.25 | <0.0001 | 67.00 | 4.45 | 0.54 |
| NIN | 6.67 | 13.67 | 9.63 | 17.65 | 10.96 | <0.0001 | 76.40 | 7.46 | 0.70 |
| INL (cm) | 19.33 | 62.13 | 46.47 | 19.75 | 15.33 | <0.0001 | 82.30 | 227.93 | 12.35 |
| SDI (cm) | 1.05 | 2.20 | 1.64 | 15.21 | 1.28 | 0.22 | 8.20 | 0.07 | 0.06 |
| PHT ‘cm) | 129.00 | 347.67 | 249.25 | 16.35 | 13.20 | <0.0001 | 79.90 | 4410.80 | 333.70 |
| NDF (days) | 66.00 | 82.00 | 75.43 | 6.14 | 21.24 | <0.0001 | 86.80 | 60.05 | 2.83 |
| PLE (cm) | 25.83 | 79.50 | 52.53 | 21.37 | 29.86 | <0.0001 | 90.40 | 361.85 | 12.23 |
| PAL (cm) | 11.67 | 41.67 | 29.19 | 21.76 | 8.59 | <0.0001 | 71.20 | 99.82 | 10.57 |
| PAW (cm) | 5.24 | 11.67 | 8.02 | 19.65 | 11.02 | <0.0001 | 76.50 | 6.42 | 0.54 |
| WPA (g) | 101.21 | 189.16 | 140.63 | 12.55 | 2.05 | 0.01 | 25.50 | 476.50 | 219.00 |
| Brix (%) | 12.80 | 24.17 | 19.12 | 13.61 | 3.53 | <0.0001 | 45.20 | 13.11 | 3.69 |
NVT: Number of vegetative tillers, NPT: Number of productive tillers, NAT: Number of aerial tillers; LEL: Leaf length, LEW: Leaf width; NIN: Number of internodes; INL: Internode length; PHT: Plant height; SDI: Stem diameter, NDF: Flowering date; PLE: Peduncle length; PAL: Main panicle length, PAW: Main panicle width, WPA: Weight of the main panicle, Pr.: Probability; F: Fisher value, CV: Coefficient of variation, Min.: Minimum, Max.: Maximum, R2: Coefficient of determination, MSG: Mean square for genotype; MSE: Mean square for error
3.3. Relationship between Agro-Physiological Traits and Brix
The Pearson correlation matrix showed significant correlations between quantitative traits. Brix was positively and moderately correlated with plant height (PHT) (r = 0.37) and peduncle length (r = 0.43) [Table 3]. Positive and strong correlations (r > 60) were recorded between the NVTs and that of productive tillers (r = 0.82), the PHT and peduncle length (r = 0.76), the number of internodes and the flowering date (r = 0.72), and the PHT and internode length (r = 0.62). However, negative and strong correlations (r > 60) were observed between SDI and each of the following traits: Internode length (r = −0.75), NVTs (r = −0.65), and number of productive tillers. Finally, the flowering date was positively correlated with SDI (r = 0.43) and negatively correlated with the weight of the main panicle (r = −0.42).
Table 3: Correlations between the quantitative agro-physiological traits and the Brix of sweet stalk sorghum genotypes.
| Traits | NVT | NPT | NDF | LEL | LEW | NIN | INL | PHT | SDI | PLE | PAL | PAW | WPA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPT | 0.82 | 1.00 | |||||||||||
| NDF | −0.20 | −0.23 | 1.00 | ||||||||||
| LEL | −0.20 | −0.23 | −0.23 | 1.00 | |||||||||
| LEW | −0.52 | −0.44 | 0.20 | 0.19 | 1.00 | ||||||||
| NIN | −0.49 | −0.40 | 0.72 | −0.14 | 0.47 | 1.00 | |||||||
| INL | 0.63 | 0.61 | −0.31 | −0.28 | −0.55 | −0.54 | 1.00 | ||||||
| PHT | 0.25 | 0.30 | 0.21 | −0.16 | −0.15 | 0.22 | 0.61 | 1.00 | |||||
| SDI | −0.65 | −0.67 | 0.43 | 0.18 | 0.38 | 0.50 | −0.75 | −0.40 | 1.00 | ||||
| PLE | 0.31 | 0.35 | −0.22 | 0.06 | −0.20 | −0.29 | 0.67 | 0.76 | −0.47 | 1.00 | |||
| PAL | 0.25 | 0.10 | −0.16 | 0.34 | −0.03 | −0.24 | 0.17 | 0.29 | −0.02 | 0.60 | 1.00 | ||
| PAW | 0.03 | −0.02 | 0.07 | 0.14 | −0.10 | −0.05 | 0.22 | 0.43 | −0.05 | 0.53 | 0.50 | 1.00 | |
| WPA | 0.20 | 0.18 | −0.42 | 0.29 | −0.24 | −0.45 | 0.32 | 0.17 | −0.30 | 0.52 | 0.62 | 0.24 | 1.00 |
| Brix | 0.02 | 0.15 | −0.26 | 0.18 | 0.09 | −0.12 | 0.27 | 0.37 | −0.30 | 0.43 | 0.23 | 0.31 | 0.19 |
NVT: Number of vegetative tillers; NPT: Number of productive tillers; NAT: Number of aerial tillers; LEL: Leaf length; LEW: Leaf width; NIN: Number of internodes; INL: Internode length; PHT: Plant height; SDI: Stem diameter; NDF: Flowering date; PLE: Peduncle length; PAL: Main panicle length; PAW: Main panicle width; WPA: Weight of the main panicle
3.4. Shannon-Weaver Diversity Index Analysis of Qualitative Morpho-Agronomical Traits
The Shannon-Weaver diversity index of four qualitative traits of the 29 sweet stalk sorghum genotypes showed high diversity index values (H’ > 0.80) of the traits related to grain characteristics such as grain coverage, grain color, and grain shape [Table 4]. The lowest Shannon-Weaver diversity index was observed for panicle exsertion with a value of 0.452.
Table 4: Shannon-Weaver diversity index of the four qualitative morpho-agronomic traits of sweet stalk sorghum genotypes.
| Traits | Class number | Shannon-Weaver diversity index (H’) |
|---|---|---|
| Exsertion | 3 | 0.452 |
| Grain shape | 2 | 0.850 |
| Grain coverage | 4 | 0.910 |
| Grain color | 4 | 0.806 |
3.5. Analysis of Genetic Parameters of Agro-Physiological Traits and Brix
The estimation of genetic parameters showed greater phenotypic variances than genotypic variances of the all studied traits [Table 5]. The extreme values of phenotypic variance were 0.02 for SDI, and 1470.27 for PHT, while those of genotypic variance were 0.01, and 1359.03 for the same traits. Six agro-physiological traits (NVT, LEW, NIN, SDI, NPT, and PAW) and Brix had phenotypic and genotypic variances below 10. All the other seven traits expressed phenotypic variances above 20. Of these traits, leaf length and flowering date had genotypic variances between 10 and 20, while the other five traits had genotypic variances above 20.
Table 5: Estimated genetic parameters of the 13 quantitative agro-physiological traits and the Brix of sweet stalk sorghum genotypes.
| Traits | δ2g | δ2p | H2(%) | GCV (%) | PCV (%) | GA | GAM (%) |
|---|---|---|---|---|---|---|---|
| NVT | 1.56 | 2.80 | 55.78 | 25.88 | 34.65 | 1.92 | 39.82 |
| NPT | 0.50 | 1.09 | 46.11 | 21.93 | 32.29 | 0.99 | 30.67 |
| LEL ‘cm) | 15.11 | 21.45 | 70.42 | 5.90 | 7.03 | 6.72 | 10.19 |
| LEW (cm) | 1.30 | 1.48 | 87.84 | 18.54 | 19.78 | 2.20 | 35.79 |
| NIN | 2.25 | 2.49 | 90.61 | 15.59 | 16.38 | 2.94 | 30.58 |
| INL (cm) | 71.86 | 75.98 | 94.58 | 18.24 | 18.76 | 16.98 | 36.55 |
| SDI (cm) | 0.01 | 0.02 | 21.64 | 4.42 | 9.50 | 0.07 | 4.24 |
| PHT ‘cm) | 1359.03 | 1470.27 | 92.43 | 14.79 | 15.38 | 73.01 | 29.29 |
| NDF (days) | 19.07 | 20.02 | 95.29 | 5.79 | 5.93 | 8.78 | 11.64 |
| PLE (cm) | 116.54 | 120.62 | 96.62 | 20.55 | 20.91 | 21.86 | 41.61 |
| PAL (cm) | 29.75 | 33.27 | 89.41 | 18.69 | 19.76 | 10.62 | 36.40 |
| PAW (cm) | 1.96 | 2.14 | 91.52 | 17.45 | 18.24 | 2.76 | 34.39 |
| WPA (g) | 85.83 | 158.83 | 54.04 | 6.59 | 8.96 | 14.03 | 9.98 |
| Brix (%) | 3.14 | 4.37 | 71.86 | 9.27 | 10.93 | 3.09 | 16.18 |
NVT: Number of vegetative tillers, NPT: Number of productive tillers, NAT: Number of aerial tillers; LEL: Leaf length, LEW: Leaf width; NIN: Number of internodes; INL: Internode length; PHT: Plant height; SDI: Stem diameter, NDF: Number of days sowing–flowering; PLE: Peduncle length; PAL: Main panicle length; PAW: Main panicle width; WPA: Weight of the main panicle, δ2p: Phenotypic variance; δ2g: Genotypic variance, H2: Broad sense heritability, √, GCV: Genotypic Coefficient of Variation, PCV: Phenotypic coefficient of variation, GA: Expected Genetic Advance, GAM: Genetic Advance as percent Mean
The broad sense heritability (H2) was 21.64% for SDI and 96.62% for peduncle length. Only SDI expressed heritability below 40%. Brix, leaf length, main panicle weight, and tillering-related traits had heritability values ranging from 40% to 80%, while the other eight agro-physiological traits including PHT, internode characteristics (NIN and INL), leaf width, flowering date, and panicle dimensions (PAL and PAW) had heritability values above 80%.
For all studied traits, the coefficients of phenotypic variation were higher than those of genotypic variation. Both coefficients were lower than 11% for Brix, leaf length, SDI, flowering date, and main panicle weight and higher than 20% for tillering-related traits (NVT, NPT) and peduncle length. The other six agro-physiological traits (LEW, NIN, INL, PHT, PAL, and PAW) showed phenotypic and genotypic coefficients of variation between 11% and 20%.
The expected genetic advance as percent mean (GAM) was 4.24% for SDI and 41.61% for peduncle length. Thus, the expected genetic advance as percent mean was below 10% for SDI and main panicle weight. GAM ranged between 10% and 20% for leaf length (10.19%), flowering date (11.64%), and Brix (16.18%) and above 20% for the other nine agro-physiological traits, leaf width, PHT, number of vegetative and productive tillers, internodes (NIN and INL), peduncle length, and panicle characteristics (PAL and PAW).
4. DISCUSSION
The large variability of evaluated germplasm is demonstrated by the significant differences observed in most quantitative traits. The high Shannon-Weaver diversity index of qualitative traits provides a diverse range of genotype choices based on desired traits and breeding program objectives. The previous studies on sorghum have reported similar results on high variability of quantitative traits [29,30] and high Shannon-Weaver diversity index of qualitative traits [31]. The Brix values of the 29 local sweet stalk sorghum genotypes (12.8–24.17%) show that they are overall sweeter than the 43 genotypes of ICRISAT (India) and Netherlands (Brix ranging between 08.30% and 16.90%) evaluated under contrasting temperate and tropical environments [32].
The high variability of qualitative traits is probably related to the high racial diversity encountered within this type of sorghum. Indeed, the five main races and several intermediate races are found in sweet stalk sorghum [14]. Moreover, this important morphological variability of accessions could be attributed to the strong differentiation of genotypes that are derived from several cycles of self-pollination.
The knowledge of the correlations allows the development of new breeding strategies using indirect selection to the extent that the phenotypic correlations are due to linked or pleiotropic genes [33]. The strong positive correlation observed between the NVTs and the number of productive tillers could be explained by the selection pressure exerted by farmers on ecotypes emitting productive tillers. Empirical selection apparently eliminated varieties emitting degenerative tillers at the bolting stage that causes unnecessary resource exports. The weak positive correlations of Brix with peduncle length and PHT may reflect a weak genetic link between these traits. There should therefore be no link between the Dw gene responsible for PHT and the gene(s) responsible (s) for sugar accumulation in sweet stalk sorghum [34]. The apparent association between sugar accumulation in the stem and PHT is thought to be the result of selection or is driven by physiological or genetic constraints [34]. Positive correlations have also been reported between PHT and Brix with respective correlation coefficients [31,34,35].
The higher the heritability of the traits, the more effective the selection. Broad sense heritability is high or very high if its value is equal to or higher than 80%, moderate between 40 and 80%, low if its value is lower than 40% [36]. Broad sense heritability was low for SDI, moderate for Brix, leaf length, main panicle weight, tillering traits (NVT and NPT), and high for the other eight agro-physiological traits including PHT, internode characteristics (NIN and INL), leaf width, flowering date, and panicle size (PAL and PAW). Several authors have reported high heritability values for the same traits [17,29,37].
The joint estimation of genotypic coefficient of variation (GCV) and heritability (H2) provides the best information for selecting parents to cross for desired traits [38]. The genotypic and phenotypic coefficients of variation (above 20% was high, 11–20% was medium, and below 11% was low) are low for Brix, leaf length, SDI, flowering date, main panicle weight, and high for tillering-related traits (NVT and NPT) and peduncle length [39,40]. The other six agro-physiological traits (LEW, NIN, INL, PHT, PAL and PAW) have moderate phenotypic and genotypic coefficients of variation. High phenotypic and genotypic coefficients of variation for PHT and main panicle yield, moderate for panicle length, and low for the flowering date have also been reported [17,41]. The small difference between genotypic and phenotypic coefficients of variation for most traits, with the exception of SDI, may indicate a very low environmental influence on these traits [42] and a higher genetic control of their expression. The high heritability of these traits confirms the weak influence of environmental factors on their expression. In this case, the phenotype allows a good prediction of the genotype of the lines [43]. Similar results were reported on sorghum [44] and pearl millet [45].
High broad-sense heritability alone does not predict that selection will provide improvement in genetic advance. The joint estimation of high broad-sense heritability and genetic advance as percent mean (GAM) can nevertheless provide more reliable information [27,46]. The genetic advance as percent mean (above 20% was high, 11–20% was medium, and below 11% was low [39]) was low for SDI and main panicle weight medium for leaf length, flowering date and Brix, and high for the other nine agro-physiological traits (LEW, PT, NVT, NPT, NIN, INL, PLE, PAL, and PAW). Seven traits including leaf width, internode length, and number, PHT, peduncle length, panicle length, and width combined high broad-sense heritability with high genetic advance as percent mean. This indicates that these traits are highly heritable and that selection for efficient genotypes is possible to improve these traits. These traits would be mainly under the control of genes with additive effects and selection can be effective in the early generations for these traits [27,47]. A direct selection method is possible for these traits. High heritability associated with a high genetic advance as percent mean was reported on panicle width, PHT, and panicle length [17,21,48]. On the other hand, high broad-sense heritability associated with a moderate genetic advance obtained for flowering date and moderate broad-sense heritability coupled with high genetic advance as percent mean of main panicle weight have been reported [17,49].
5. CONCLUSIONS
There was high variability of the agro-morphological and physiological traits and relatively high Brix values in local sweet stalk sorghum genotypes. Positive correlations of Brix with PHT and stalk length were observed. In addition, a high Shannon-Weaver diversity index for qualitative traits and high broad sense heritability associated with high genetic advance as percent mean for seven agro-physiological traits were found. Small differences between genotypic and phenotypic coefficients of variation were also recorded for most quantitative traits. These results, which show possibilities for improvement by direct selection, could be strengthened by a better estimation of the GXE effect on trait expression through multi-location and multi-year trials.
6. ACKNOWLEDGMENT
The authors are grateful to the AVISA project funded by the Bill and Melinda Gate Foundation and coordinated by CIMMYT for supporting the publication of this paper. We sincerely thank the “Laboratoire Biosciences” of the “Université Joseph Ki-Zerbo” for its support in carrying out the study. We are grateful to Prof Vernon E. Gracen of the West African Center for Crop Improvement (WACCI) in Ghana and Cornell University for his valuable help in correcting the English language. We pay tribute to the late Dr. Boureima Sawadogo for his help in designing the research protocol and monitoring the student during the experiment.
7. AUTHORS’ CONTRIBUTIONS
All the authors were substantially involved in the production of this manuscript. NS designed the experiment, performed the data analysis and interpretation, and drafted the manuscript. ID and NO participated in the design of the experiment, the analysis, and interpretation of the data and the manuscript reading. WHT, TLKB, and JS monitored the trial, collected the data, and contributed to the analysis of the data and reading of the manuscript. GC read and corrected the manuscript and ensured its translation into English, MHO and KRN contributed to the correction of the manuscript, and PBK supervised the whole work.
8. FUNDING
The research was funded by the authors.
9. CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest regarding the publication of this paper.
10. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
11. DATA AVAILABILITY
The data supporting the findings of this study are available from the corresponding author on request.
12. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Duval, A, Mijatovic, D, Hodgkin, T. The Contribution of Biodiversity for Food and Agriculture to the Resilience of Production Systems-thematic Study for the State of the World's Biodiversity for Food and Agriculture. Rome:Food and Agriculture Organization;2019. 85.
2. WWF. Farming with Biodiversity. Towards Nature-positive Production at Scale. Gland, Switzerland:WWF International;2021. 52.
3. DGPER. National Strategy for Rice Development [Stratégie Nationale de Développement de la Riziculture]. Ouagadougou/Burkina Faso:MAAH;2011. 43.
4. FAO/GIEWS. GIEWS Country Brief Burkina Faso;2022. Available from https://www.fao.org/giews/countrybrief/country/BFA/pdf_archive/BFA_Archive.pdf [Last accessed on 2022 Aug 05].
5. Mindaye TT, Mace ES, Godwin ID, Jordan DR. Heterosis in locally adapted sorghum genotypes and potential of hybrids for increased productivity in contrasting environments in Ethiopia. Crop J 2016;4:479-89. [CrossRef]
6. FAOSTAT. Statistical Yearbook. World Food and Agriculture, Organization UN;Rome, Italy;2021. Available from:https://www.faostat.fao.org/site/291/default.aspx [Last accessed 2022 Aug 05].
7. USDA. World Agricultural Production. Foreign Agricultural Service/USDA 2 July 2022 Global Market Analysis;2022. 43. Available from:https://www.production.pdfusda.gov [Last accessed 2022 05 Aug].
8. Holou RA, Stevens G. Juice, sugar, and bagasse response of sweet sorghum (Sorghum bicolor (L.) Moench cv. M81E) to N fertilization and soil type. GCB Bioenergy 2012;4:302-10. [CrossRef]
9. Rolz C, de León R, Ana Luisa MD. Co-production of ethanol and biodiesel from sweet Sorghum juice in two consecutive fermentation steps. Electron J Biotechnol 2019;41:13-21. [CrossRef]
10. Klasson Kt, Boone SA. Bioethanol fermentation of clarified sweet sorghum (Sorghum bicolor (L.) Moench) syrups sealed and stored under vegetable oil. Ind Crops Prod 2021;163:113330. [CrossRef]
11. Ray RC, Uppuluri KB, Trilokesh C, Lareo C. Sweet Sorghum for bioethanol production:Scope, technology, and economics. In:Ray RC, Ramachandran S, editors. Bioethanol Production from Food Crops. New York:Academic Press;2019, 81-100. [CrossRef]
12. Lavudi S, Oberoi HS, Mangamoori LN. Ethanol production from sweet Sorghum bagasse through process optimization using response surface methodology. 3 Biotech 2017;7:233. [CrossRef]
13. Sawadogo N, Tiendrebéogo J, Naoura G, BéréTL, Tonde WH, Sawadogo B, et al. Photoperiod sensitivity and variability of agromorphological traits and brix content of sweet Sorghum cultivated in Burkina Faso under two sowing dates. Adv Agric 2022;2022:1-10. [CrossRef]
14. Sawadogo N, Naoura G, Ouoba A, Yaméogo N, Tiendrebeogo J, Ouédraogo MH. Phenotypical characteristics and genetic diversity of three types of Sorghum [Sorghum bicolor (L.) Moench] cultivated in Burkina Faso based on qualitative traits. Moroccan J Agric Sci 2022;3:109-16. [CrossRef]
15. Sawadogo N, Ouédraogo MH, Bougma LA, Yaméogo N, TondéWH, Tiendrébéogo J, et al. Assessment of genetic variability of three types of Sorghum cultivated in Burkina Faso using morphoagronomic quantitative traits and brix. In:Çal??kan M, Aydin S, editors. Genetic Diversity-recent Advances and Applications. London:IntechOpen;2022, 1-16. Available from:https://www.intechopen.com/online-first/82862 [Last accessed 2022 Jul 31].
16. Tiendrebéogo J, Sawadogo N, KiébréM, KiébréZ, Tuina S, Sawadogo TA, et al. Genetic relationship between sweet grain Sorghum and the other Sorghum types cultivated in Burkina Faso assessed with nuclear microsatellite markers SSRs. Am J Plant Sci 2022;13:872-83. [CrossRef]
17. Gebregerg G, Mekbib F, Moral MT. Estimation of genetic variability, heritability, and genetic advance in advanced lines for grain yield and yield components of Sorghum [Sorghum bicolor (L.) Moench] at Humera, Western Tigray, Ethiopia. Cogent Food Agric 2020;6:1764181. [CrossRef]
18. Jimmy ML, Nzuve F, Flourence O, Manyasa E, Muthomi J. Genetic variability, heritability, genetic advance, and trait correlations in selected Sorghum (Sorghum bicolor L. Moench) varieties. Int J Agron Agric Res 2017;11:47-56.
19. Mary SS, Gopalan A. Dissection of genetic attributes yields traits of fodder cowpea in F3 and F4. J Appl Sci Res 2016;2:805-8.
20. Badran AE. Genetic parameters of some sorghum (Sorghum bicolor (L.) Moench) genotypes under water deficit stress. Egypt J Desert Res 2020;70:103-19. [CrossRef]
21. Kalpande HV, More AW, Aundhekar RL, Dhutmal RR. Genetic variability, heritability and genetic advance in sweet grain (Hurda) Sorghum [Sorghum bicolor (L.) Moench]. Int J Curr Microbiol Appl Sci 2018;6:400-5.
22. Latif NA, Nain FN, Malim NH. Abdullah R, Rahim MF, Mohamad MN, et al. Predicting heritability of oil palm breeding using phenotypic traits and machine learning. Sustainability 2021;13:12613. [CrossRef]
23. Rao MR, Patil SJ. Variability and correlation studies in F2 population of Kharif x rabi crosses of Sorghum. Karnataka J Agric Sci 1996;9:78-84.
24. Mahajan RC, Wadikar PB, Pole SP, Dhuppe MV. Variability, correlation, and path analysis studies in Sorghum. Res J Agric Sci 2011;2:101-3.
25. BUNASOLS. Pedological Study in the Experimental Station of Gampèla. Scale 1/5000, Technical Report 59;2019. 53.
26. Gashaw ET, Mekbib F, Ayana A. Genetic diversity among Sugarcane genotypes based on qualitative traits. Adv Agric 2016;2016:1-8. [CrossRef]
27. Johnson HW, Robinson H, Comstock RE. Estimates of genetic and environmental variability in soybeans. Agron J 1955;47:314-8. [CrossRef]
28. Ahsan MZ, Majidano MS, Bhutto H, Soomro AW, Panhwar FH, Channa AR, et al. Genetic variability, coefficient of variance, heritability and genetic advance of some Gossypium hirsutum L. J Agric Sci 2015;7:147-51. [CrossRef]
29. Tomar SS, Sivakumar S, Ganesamurthy K. Genetic variability and heritability studies for different quantitative traits in sweet Sorghum [Sorghum bicolor (L.) Moench] genotypes. Electron J Plant Breed 2012;3:806-10.
30. Lombardi GM, Nunes JA, Parrella RA, Teixeira DH, Bruzi AT, Durães NN, et al. Path analysis of agro-industrial traits in sweet sorghum. Genet Mol Res 2015;14:16392-402. [CrossRef]
31. Shegro A, Labuschagne MT, Geleta N, Van Biljon A. Assessment of genetic diversity in sorghum using phenotypic markers. Cereal Res Commun 2013;41:509-18. [CrossRef]
32. Udoh DA, Rasmussen SK, Jacobsen SE, Iwo GA, de Milliano W. Yield stability of Sweet Sorghum genotypes for bioenergy production under contrasting temperate and tropical environments. J Agric Sci 2018;10:42-53. [CrossRef]
33. Falconer DS, Mackay TF. Introduction to Quantitative Genetics. 4th ed. Edinburgh:Longman Group Limited;1996.
34. Shukla S, Felderhoff TJ, Saballos A, Vermerris W. The relationship between plant height and sugar accumulation in the stems of sweet Sorghum (Sorghum bicolor (L.) Moench). Field Crops Res 2017;203:181-91. [CrossRef]
35. Burks PS, Kaiser CM, Hawkins EM, Brown PJ. Genomewide association for sugar yield in sweet Sorghum. Crop Sci 2015;55:2138-48. [CrossRef]
36. Singh BD. Plant Breeding:Principles and Methods. New Delhi, India:Kalyani Publishers;2001. 896.
37. Mofokeng MA, Shimelis H, Laing M, Shargie N. Genetic variability, heritability, and genetic gain for quantitative traits in South African Sorghum genotypes. Aust J Crop Sci 2019;13:1-10. [CrossRef]
38. Hamidou M, Souley AK, Kapran I, Souleymane O, Danquah EY, Ofori K, et al. Genetic variability and its implications on early generation Sorghum lines selection for yield, yield contributing traits, and resistance to Sorghum midge. Int J Agron 2018;2018:1-10. [CrossRef]
39. Kakeeto R, Baguma SD, Subire R, Kaheru J, Karungi E, Biruma M. Genetic variation and heritability of kernel physical quality traits and their association with selected agronomic traits in groundnut (Arachis hypogeae) genotypes from Uganda. Afr J Agric Res 2019;14:597-603. [CrossRef]
40. Khan MM, Rafii MY, Ramlee SI, Jusoh M, Oladosu Y, Al Mamun M, et al. Unveiling genetic diversity, characterization, and selection of Bambara Groundnut (Vigna subterranea L. Verdc) genotypes reflecting yield and yield components in tropical Malaysia. Biomed Res Int 2022;2022:6794475. [CrossRef]
41. Warkad YN, Potdukhe NR, Dethe AM, Kahate PA, Kotgire RR. Genetic variability, heritability, and genetic advance for quantitative traits in Sorghum germplasm. Agric Sci Dig 2008;28:165-9.
42. Alam MA, Rahman M, Ahmed S, Jahan N, Khan MA, Islam MR, et al. Genetic variation and genotype by environment interaction for agronomic traits in Maize (Zea mays L.) Plants (Basel) 2022;11:1522. [CrossRef]
43. Hussain SA, Iqbal MS, Akbar M, Arshad N, Munir S, Ali MA, et al. Estimating genetic variability among diverse lentil collections through novel multivariate techniques. PLoS One 2022;17:e0269177. [CrossRef]
44. Khan MM, Rafii MY, Ramlee SI, Jusoh M, Mamun A. Genetic variability, heritability, and clustering pattern exploration of Bambara Groundnut (Vigna subterranea L. Verdc) accessions for the perfection of yield and yield-related traits. Biomed Res Int 2020;2020:2195797. [CrossRef]
45. Drabo I, ZangréRG, Sawadogo M, Ouedraogo M. Genetic variability and estimates of genetic parameters in Burkina Faso's pearl millet landraces. Int J Agric Forest 2013;3:367-73.
46. Anoumaa M, Kouam EB, Kanmegne G, Kamga YB, Sime HD, Nkombo LL, et al. Phenotypic variation and genetic divergence studies in Cameroonian potato (Solanum tuberosum L.) genotypes. J Appl Biol Biotech 2023;11:210-21.
47. Konate AK, Zongo A, Kam H, Sanni A, Audeber A. Genetic variability and correlation analysis of rice (Oryza sativa L.) inbred lines based on agro-morphological traits. Afr J Agric Res 2016;11:3340-6. [CrossRef]
48. Verma LK, Biradar BD. Genetic variability among B and R lines on milo and maldandi cytoplasm across the years in rabi Sorghum [Sorghum bicolor (L.) Moench]. Electron J Plant Breed 2022;13:1132-6. [CrossRef]
49. Dyulgerova B, Valcheva D. Heritability, variance components and genetic advance of yield and some yield related traits in barley doubled haploid lines. Türk Tar?mve Do?a Bilimleri Dergisi 2014;1:614-7.