1. INTRODUCTION
Strobilanthes (Family: Acanthaceae) is a South-East Asian wet tropical evergreen genus that exists in moist forests. This genus with 400 species is the second largest in the family Acanthaceae [1], and distributed along the South and Southeast Asia. It reached India after the Indian subcontinent merged Asian mainland through the continental drift. The original genetic stock of Strobilanthes was probably a single species, reached from Malaysian region to the Western Ghats [2]. In India, there are 146 species of Strobilanthes distributed in the wet non-deciduous forests of the Western Ghats and the Himalayas [3]. Fifty-nine species are reported from South India with maximum endemism in Peninsular India [4]. Beddome incompletely described the species Strobilanthes andersonii Bedd in 1864 [5]. Due to the long flowering periodicity, no further collections of this plant were made making the description of this plant incomplete and no further attempt was made on revising the morphology till now. Furthermore, since then, this species was considered extinct [4]. The massive flowering of this species was noticed in the shola forests of Eravikulam National Park in 2008 (2). Pendent flowering spikes with large green bracts were the distinguishing feature of this species. In the present study, the gregarious flowering of S. andersonii Bedd is noticed in 2018 in the same locality. Hence, the flowering periodicity of this endemic shrub is ascertained as 10 years.
The recent collection of this species in flowering stage from Eravikulam National Park became a rediscovery of this species. Even though there was previous plant exploration in the same area (Eravikulam NP), there were no report of its existence. It shows that even though the plant flowered according to its own reproductive cycle the botanists who explored this area could not collect it in flowering stage. Furthermore, there was a note on previous reports indicating the huge collection of Strobilanthes in vegetative parts [6,7]. This urges a new attempt to identify the species of Strobilanthes using the foliar characters. Augustine [2] has proposed a key based on the foliar characters of Strobilanthes. The revised morphology of this species based on the recent data is given in this paper. However, a more precise mechanism is the utilization of molecular techniques for identifying the vegetative stages.
DNA Barcoding uses short DNA sequences from nuclear and organelle genomes for the identification of biological specimens [8]. The difference between inter and intra-specific genetic distances within a group of organisms is DNA Barcoding gap [9]. DNA Barcoding can resolve taxonomic and evolutionary problems related to identification [10-12]. In DNA Barcoding, the basic concept is to discriminate the species by the variation in the DNA sequences. In species like Strobilanthes, where long flowering periodicity exists, DNA Barcoding is a suitable method for identifying already described species and describing new species.
Nuclear ribosomal DNA (nr DNA) contains 18S, 5.8S, and 26S ribosomal RNA subunit and different spacer DNA regions in between [13]. In the present study, molecular phylogenetic elucidation of S. andersonii Bedd and related species of Strobilanthes was done using ITS nr DNA. Hemigraphis confinis T. Anderson as selected the out-group.
2. MATERIALS AND METHODS
2.1. Taxonomy
First description S. andersonii Bedd. Madras J.Lit. and Sci. 1:55, 1864 [5].
2.2. Habitat and Distribution
Evergreen shola forests, 2000 m above the sea level, Kerala, India. The present collection is from Eravikulam National Park, Idukki District, Southern Western Ghats, India.
2.3. Materials and Methods
S. andersonii used in the study was collected from the evergreen shola forests, Eravikulam National Park, Idukki District, and Kerala in November 2018. The plant species were collected and brought to the laboratory for further molecular analyses. The collected plant specimen, the voucher samples were deposited in the Calicut University Herbarium (CALI) Kerala (Voucher Specimen Number – 7068), (Collection Number – 2101).
2.4. DNA Extraction
The total genomic extraction was done with Nucleospin Plant II kit (Mechery-Nagel) using 100 mg of fresh leaves homogenized using liquid nitrogen. The quality of the isolated DNA was tested by agarose gel electrophoresis followed by gel staining with ethidium bromide.
2.5. Polymerase Chain Reaction (PCR) Amplification and Sequencing
PCR amplifications of the ITS region were carried out in a PCR thermal cycler [14] using the primer pairs [15] and thermo cycling conditions given in Table 1.
Table 1: Primers and reaction conditions.
| DNA region | Primer pairs | Primer sequence (5-3) | Thermo cycling conditions |
|---|---|---|---|
| ITS | ITS-5F ITS-4R | GGAAGTAAAAGTCGTAACAAGG TCCTCCGCTTATTGATATGC | 98°C 30 s, 98°C 5 s, 58°C 10 s, 72°C 15 s, 72°C 60 s, 4°C-40 cycles |
Sequencing reaction was performed with the Big Dye Terminator v3.1 cycle sequencing kit [14]. Sequence Scanner Software v1 was used for checking the quality of the sequence (Applied Bio system). Analysis and comparison of the locus were done with the help of BLAST routines [16].
2.6. Phylogenetic Analysis
An ITS phylogram was constructed using ML analysis performed by RAxML with default parameters ans 1000 bootstrap replications [17,18]. The criterion used to assess BS support percentages (BP) was as follows: low 50–70%, moderate 71–84%, and strong 95–100. The in group taxa consisted of S. andersonii Bedd and 40 other species of Strobilanthes retrieved from NCBI Gen Bank [Table 2]. H. confinis T. Anderson was the out-group.
Table 2: List of Plant names, accession number, and collection place of Strobilanthes species, retrieved from NCBI Gen Bank.
| S. No. | Species Name and Nucleotide No. | GenBank No. ITS | Native Range |
|---|---|---|---|
| 1 | Strobilanthes andersonii Bedd. 608 | MT571642.1 | Eravikulam National Park, Southern Western Ghats, India |
| 2 | Strobilanthes kunthiana (Nees) T.Anderson 674 | AY489377.1 | Southern Western Ghats, India |
| 3 | Strobilanthes ciliate Nees 674 | AY489381.1 | Western Peninsular India |
| 4 | Strobilanthes barbata Nees 673 | AY489382.1 | Southern Western Ghats, India |
| 5 | Strobilanthes neilgherrensis Bedd 676 | AY489373.1 | Southern Western Ghats, India |
| 6 | Strobilanthes pulneyensis Clarke 628 | AY489374.1 | Southern Western Ghats, India |
| 7 | Strobilanthes lawsonii Gamble 675 | AY489375.1 | Southern Western Ghats, India |
| 8 | Strobilanthes stenodon C.B.Clarke 673 | AY489372.1 | India |
| 9 | Strobilanthes andamanensis Bor 675 | AY489386.1 | Andaman |
| 10 | Strobilanthes lupulina Nees 675 | AY489397.1 | Southern Western Ghats, India |
| 11 | Strobilanthes decurrens Nees 675 | AY489390.1 | Southern Western Ghats, India (TN) |
| 12 | Strobilanthes micrantha Wight 675 | AY489388.1 | Southern Western Ghats, India |
| 13 | Strobilanthes asper Venu& P.Danie l675 | AY489399.1 | SW.India |
| 14 | Strobilanthes anceps Nees 677 | AY489395.1 | India |
| 15 | Strobilanthes punctata Nees 678 | AY489396.1 | India |
| 16 | Strobilanthes rubicunda (Nees) T Anderson 674 | AY489370.1 | Southern Western Ghats, India |
| 17 | Strobilanthes involucrate (Blume) Bremek 676 | AY489358.1 | Java |
| 18 | Strobilanthes anceps Nees 664 | JX443807.1 | S. India, Sri. Lanka |
| 19 | Strobilanthes steenisiana J.R.Benn 676 | AY489357.1 | Java |
| 20 | Strobilanthessp. “Hongkong Island” 675 | AY489355.1 | Hongkong |
| 21 | Strobilanthes walker Arn.ex Nees 674 | AY489391.1 | Southern Western Ghats, India |
| 22 | Strobilanthes filiformis Blume 673 | AY489353.1 | Java |
| 23 | Strobilanthes imbricate Nees 672 | AY489362.1 | Indo-China |
| 24 | Strobilanthes bibracteata Blume 675 | AY489359.1 | Java |
| 25 | Strobilanthes isophylla (Nees) T.Anderson 674 | AY489352.1 | Bhutan to Bangladesh |
| 26 | Strobilanthes cernua Blume 662 | AY489361.1 | Java |
| 27 | Strobilanthes japonica (Thunb) Miq. 675 | AY489356.1 | Japan, East Asia |
| 28 | Strobilanthes alata Nees 678 | AY489360.1 | Java |
| 29 | Strobilanthes repanda (Blume) J.R.Benn 679 | AY489366.1 | Java |
| 30 | Strobilanthes pluriformis C.B. Clarke 639 | AY489346.1 | Philippines |
| 31 | Strobilanthes aprica var. pedunculata Craib 673 | AY489345.1 | South China to Indo-China |
| 32 | Strobilanthes habracanthoides J.R.I Wood 663 | AY489369.1 | India |
| 33 | Strobilanthes capitata (Nees) T.Anderson 650 | AY489349.1 | India |
| 34 | Strobilanthes versicolor Diels 699 | MT914278.1 | Tibet to China |
| 35 | Strobilanthes speciosa Blume 643 | AY489348.1 | Java |
| 36 | Strobilanthes oligocephala T. Anderson ex C.B. Clark 626 | AY489351.1 | Tibet and Assam |
| 37 | Strobilanthes dyeriana (Mast.) J.R.I.Wood 644 | AY489367.1 | Java |
| 38 | Strobilanthes attenuata Nees 643 | AY489344.1 | India |
| 39 | Strobilanthes multidens C.B.Clark 633 | AY489350.1 | India |
| 40 | Strobilanthes galeopsis Stapf 626 | AY489354.1 | Borneo |
| 41 | Strobilanthes pulcherrima T.Anderson 663 | AY489368 | Sri Lanka |
| 42 | Hemigraphis confinis T.Anderson 680 | AY489400.1 | Java |
3. RESULTS AND DISCUSSION
S. andersonii Bedd is considered to be an extinct species (4), but it is rediscovered recently (2). The flowering periodicity of this plant is 10 years. Hence, identification, discrimination, and morphological characterization for the purpose of taxonomic studies were difficult. The S. andersonii Bedd with long flowering periodicity and monocarpic nature is also a possible cause for their rarity. It is difficult to get the specimens of Strobilanthes in flowers. Hence, identification of these species is very challenging in many flora works. Hence, it results in the poor documentation of the diversity of Strobilanthes, even though the species is there in its vegetative condition. The characteristics of leaves and other vegetative parts have less importance in species wise diagnostic characteristics. Using ITS nrDNA barcoding, we can expose the genetic relationships among accessions more effectively and accurately [8].
3.1. Revised Morphology
The previous description of this species [5] was incomplete with respect to the floral characteristics which were redressed in the present revised morphology which is elaborated here [Figure 1].
 | Figure 1: Strobilanthes andersonii Bedd. (a) Habit, (b) One spike inflorescence (c) One flower longitudinally sectioned. [Click here to view] |
S. andersonii Bedd is a large shrub, growing to 4 m height; young stems angled, hirsute, terete when mature. Leaves simple, opposite; lamina 15–22 × 8–12 cm, ovate, round at base, acuminate at apex, and hirsute-hairy on upper and lower surface; acumen 2–3 cm long, pointed at apex; margin regularly crenate-serrate, ciliate; lateral nerves 6–7 pairs, alternate, regular; intercostae parallel, nervules reticulate, all prominently projected below, and impressed above; and petioles 1.5–4.5 cm long, hirsute-hairy, and hairs light pink. Inflorescences spikes, terminal and upper axillary, usually pendent, 4–7 cm long, 2–2.5 cm broad, cylindrical to obtusely sub-4-angled; bracts many, arranged in four rows, 1.5–2.5 cm across, ovate-orbicular, concave, pale green to pale purple with green-purple nerves, glabrous, margin finely serrulate; serrulations produced in to small spinules; and bracteoles 2, oblanceolate, acute at apex, 1.5 × 0.7 cm, glabrous, greenish white with green nerves, ciliate along the margins. Flowers 15–30 in each spike, densely packed, usually a pair of two opposite flowers or rarely two pairs bloom together; sepals 1.6 cm long, linear-lanceolate, acute, glabrous, ciliate along the margin, pale green; corolla 2.1 cm long, 2.5 cm across, sub-campanulate; tubular below, broadened above; tubular lower part cylindrical, 0.7 cm long, deep violet inside, glabrous; upper portion sub-campanulate, 1.4 cm broad, white-pale blue with deep violet prominent nerves, glabrous; lobes equal, orbicular, round to sub-cordate at apex, entire or shallowly crenate along the margins, glabrous, pale blue with thin blue nerves, spreading, twisted; stamen 4, free; filaments unequal, in 2 pairs, attached together into a small sheath just above the tubular part of the corolla tube; longer filaments 18 mm long, 1.5 mm thick, violet below, white above, glabrous; shorter filaments 12 mm long, inner, similar to the outer long filaments; anthers similar, just at the mouth of the corolla, oblong, shallowly cleft to sagittate at base, 5 mm long, pale pink, anthers of long stamens open first; ovary on a disc, 4 mm long, ovoid, glabrous; ovules 4, style 2.1 cm long, slender, glabrous, pink; stigma pointed into a white spot; and disk present below the ovary, 2 mm thick, yellow. Fruit 2 × 0.7 cm, ovoid, acute, glabrous; seeds four, orbicular, and compressed.
3.2. Phylogenetic Analysis
Presently, the phylogram based on ITS nr DNA gene sequences grouped all available 40 species into two clades [Figure 2]. The Strobilanthes species of Peninsular India are distributed in both. These clades were further divided into sub clades. Clade I consisted of 9 taxa divided into two groups (BS 100%). S. andersonii Bedd is coming under Group A. Group B contains 8 taxa with BSS of 51%. The species coming under group B are Strobilanthes pulneyensis Clark, Strobilanthes barbata Nees, Strobilanthes lawsonii Gamble, Strobilanthes neilgherrensis Bedd, Strobilanthes ciliata Nees, Strobilanthes kunthiana (Nees) T. Anderson, Strobilanthes stenodon C. B. Clark, and Strobilanthes andamanensis Bor. Among these, all species except S. andamanensis Bor is native to Southern Western Ghats, India. S. andamanensis Boris is from Andaman Islands. The Indian Strobilanthes species S. andersonii Bedd, S. pulneyensis Clark, S. barbata Nees, S. lawsonii Gamble, S. neilgherrensis Bedd, Strobilanthes ciliate Nees, S. kunthiana (Nees) T. Anderson, S. stenodon C. B. Clark, and S andamanensis Bor formed the most recent clade I with high boot strap support (BSS of 100%). The members of these clade can be considered as recent radiations and with Clade II as a sister clade.
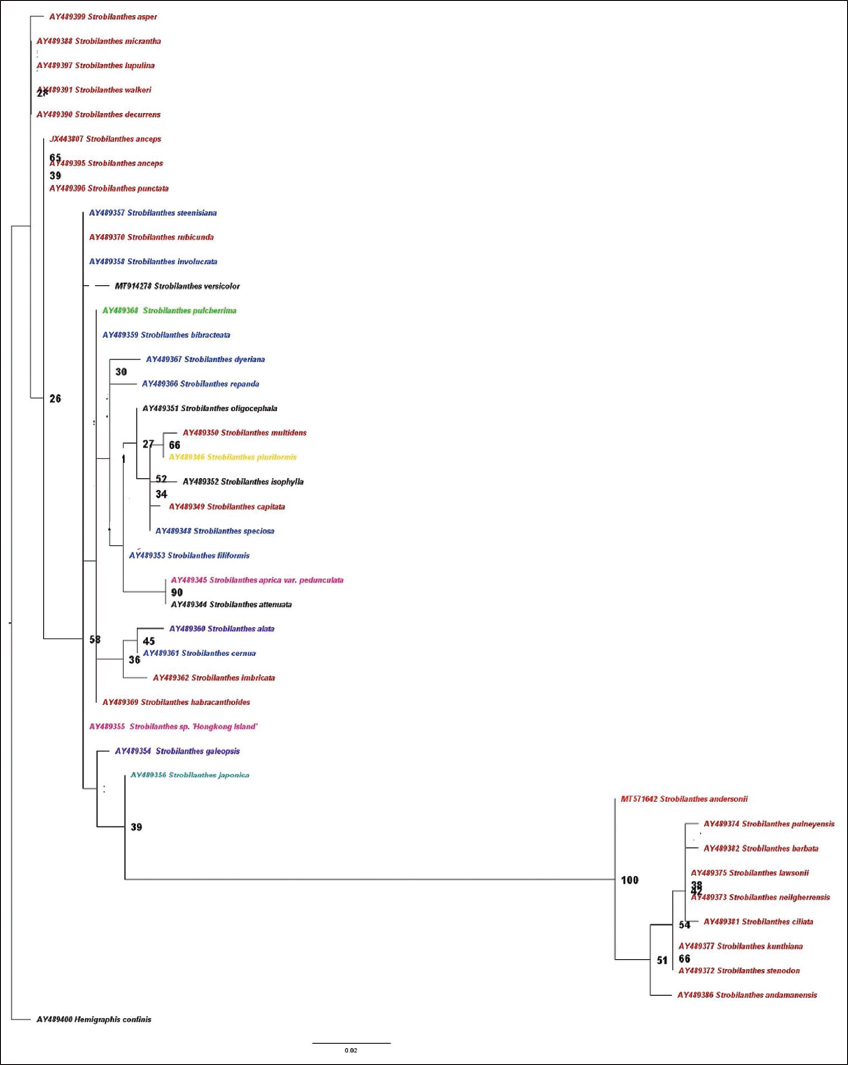 | Figure 2: ML tree inferred with RAxML of nr DNA (ITS) of Strobilanthes andersonii Bedd compared with 40 species of Strobilanthes. [Click here to view] |
Clade II consisted of 32 species and was divided into five sub-groups. Group A contains Strobilanthes japonica, the native of Japan, East Asia with BSS 39%. Group B contains Strobilanthes galeopsis Stapf is from S.E. Asia with BSS 20%. Group C contains 22 species with BSS 58%.
Group C contains species such as Strobilanthes steenisiana J.R. Benn, Strobilanthes rubicunda Nees T. Anderson, Strobilanthes involucrate (Blume) Bremik, Strobilanthes versicolor Diels, Strobilanthes pulcherrima T. Anderson, Strobilanthes bibracteata Blume, Strobilanthes dyeriana (Mast) J.R.I Wood, Strobilanthes repanda Blum J.R. Benn, Strobilanthes oligocephala T. Anderson ex C. B. Clark, Strobilanthes multidens C. B. Clark, Strobilanthes pluriformis C. B. Clark, Strobilanthes isophylla (Nees) T. Anderson, Strobilanthes capitata Nees T. Anderson, Strobilanthes speciosa Blume, Strobilanthes filiformis Blume, Strobilanthes aprica var. pedunculata Craib, Strobilanthes attenuate Nees, Strobilanthes alata Nees, Strobilanthes cernua Blume, Strobilanthes imbricate Nees, Strobilanthes habracanthoides J. R. I. Wood, Strobilanthes spp. “Hong Kong Island.” In these species S. rubicunda Nees T. Anderson, S. multidens C.B. Clark, S. capitata Nees T. Anderson, S. attenuate Nees, S. imbricate Nees, and S. habracanthoides J.R. I. Wood which are from India. S. steenisiana J.R.Benn, S. involucrate (Blume) Bremik, S. bibracteata Blume, S. dyeriana (Mast) J.R.I Wood, S. repanda Blum J. R.Benn, S. speciosa Blume, S. filiformis Blume, S. alata Nees, and S. cernua Blume are native of Java. The Tibetan species is S. versicolor Diels, S. oligocephala T. Anderson ex C, B. Clark and S. aprica var. pedunculata Craib. The Group C represents taxa from geographically diverse regions of India, Java, and Tibet forming a weakly supported group (BSS 58%).
Group D contains three species, two species of Strobilanthes anceps Nees and Strobilanthes punctate Nees which are from South Asia BS value 26%.
Group E contains five species. They are Strobilanthes asper Venu and Daniel, Strobilanthes micrantha Wight, Strobilanthes lupulina Nees, Strobilanthes walkeri Arn. ex Nees, and Strobilanthes decurrens Nees. These species are native of Southern Western Ghats, India. S. japonica is the sister taxa to this clade. Clade II is the basal clade with taxa showing an early establishment and wide geographical distribution. Members of this clade might have been the earliest radiations in the Sothern Western Ghats from an ancestor with South Asian origin.
S. andersonii Bedd and its related species were descended from clade II and recently established in Western Ghats due to adaptive radiation. In evolutionary radiation, a fast escalation in the diversity of a group of organisms occurs by frequent speciation within a particular clade with fast evolutionary and ecological divergence in geographical location [19]. S. andersonii Bedd has close affinity with other Indian species. Moreover, the analysis shows that South Indian species belong to recent linages of Strobilanthes.
4. CONCLUSION
By revisiting the taxonomic description of this species hitherto considered as extinct, a proper key for identifying the species can be formulated for field identification of the species. Furthermore, it will accelerate the conservation efforts of this species with high endemism by demarcating the areas of its occurrence a further clarification on the taxonomic ambiguity was attempted by the phylogenetic study. Internal transcribed spacer nuclear DNA sequences were suitable in taxonomic studies of Strobilanthes even if the flowers are not accessible. In the present study, sequence analysis of S. andersonii Bedd using ITS sequences was performed to clarify phylogenetic relationships and found that this species is recent radiation in the South Western Ghats.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
6. FUNDING
Department of Science and Technology, Government of India for financial support vide Ref. No SR/WOS-A/LS-396/2017 under Women Scientist Scheme (WOS-A).
7. CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
8. ETHICAL APPROVALS
Forest Department of Kerala, India providing permission to collect the plant sample and this study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
Plant sample information and data available in Table:2.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Wood JR, Scotland RW. New and little-known species of Strobilanthes (Acanthaceae) from India and Southeast Asia. Kew Bull 2009;64:3-47. [CrossRef]
2. Augustine J. Strobilanthes Blume in the Western Ghats. Kozhikkode:Malabar Natural History Society;2018.
3. Karthikeyan S, Sanjappa M, Moorthy S. Flowering Plants of India- Dicotyledons (Acanthaceae - Avicenniaceae). Vol. 1. Kolkata:Botanical Survey of India;2009. 41-60.
4. Venu P. Strobilanthes Blume (Acanthaceae) in Peninsular India. Vol. 4. Kolkata:Botanical Survey of India - Flora of India Series;2006. 12–216.
5. Beddome RH. Contributions to the Botany of Southern India. Madras J Lit Sci 1864;3:55.
6. Karunakaran PV, Rawat GS, Uniyal VK. Ecology and Conservation of the Grasslands of Eravikulam National Park, Western Ghats. Delhi:Wildlife Institute of India;1998.
7. Biju SD. Floristic Studies on Eravikulam National Park. TBGRI Research Report. Thiruvananthapuram, Kerala:Troppical Botanical Garden and Research Institute;2004.
8. Natascha T, Pan Z, Praveen I. DNA barcoding of medicinal plant material for identification. Curr Opin Biotechnol 2014;25:103-10. [CrossRef]
9. Hebert PD, Cywinska A, Ball SL, de Waard JR. Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci 2003;270:313-21. [CrossRef]
10. Kress WJ, Wurdack KJ, Zimmer EA, Weight LA, Janzen DH. Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci 2005;102:8369-74. [CrossRef]
11. Kress WJ, Erickson DL. A two-locus global DNA barcode for land plants:The coding rbcL gene complements the non-coding trn H-psbA region. PLoS One 2007;2:e508. [CrossRef]
12. Miller SE. DNA barcoding and the renaissance of taxonomy. Proc Natl Acad Sci U S A 2007;104:434-9. [CrossRef]
13. Polans NO, Saar DE, Sorensen PD. A phylogenetic analysis of the genus Dahlia (Asteraceae) based on Internal and External Transcribed spacer regions of nuclear ribosomal DNA. Syst Bot 2003;28:627-39.
14. ABI PRISM Big Dye Terminator v3.1 Cycle Sequencing Kit-User Manual. California 94404,USA:Applied Bio Systems;2002.
15. White TJ, Bruns TD, Lea SB, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In:Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols:A Guide to Methods and Applications. New York:Academic Press, Inc.;1990. 315-22. [CrossRef]
16. Available from:http://www.ncbi.nih.gov/BLAST.2/10/2021
17. Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 2008;75:758-71. [CrossRef]
18. Stamatakis A. RAxML-VI-HPC:Maximum likelihood based phylogenetic analysis with thousands of taxa and mixed models. Bioinformatics 2006;22:2688-90. [CrossRef]
19. Schenk JJ. The Next generation of adaptive radiation studies in plants. Int J Plant Sci 2021;182:245-62. [CrossRef]