1. INTRODUCTION
Probiotics have gained significant research consideration in recent years because of their multiple health benefits [1]. The word “probiotic” came from the Greek word “pro-bios” which literally means “for life.” Probiotics are microbial cell which when supplemented in an adequate amounts, confer beneficial effects on the health, and well-being of the consumers [2]. Health benefits of probiotic organisms are highly specific with regard to species and strains and it cannot be generalized. Therefore, bio-prospecting for novel strains of efficient probiotics has been continuously practiced [1].
There are some basic selection criteria for probiotics to be used as food supplement, that is, its safety, viability/activity in delivery carriers, acid and bile resistance, adherence and colonization in gastro-intestinal tract, and production of antimicrobial substances such as bacteriocin [3,4]. Probiotics also possess some special properties such as anti-inflammatory, anti-oxidant, anti-diabetic, anti-cancer, cholesterol removal, and vitamin production [5]. Indeed, several studies have validated that probiotics have advantageous effects on a wide range of disorders such as irritable bowel syndrome, inflammatory bowel disease, diarrhea, constipation, hypertension, lactose intolerance, and hypersensitivity [6].
In recent years, due to the food habits, people are suffering more with several cardiovascular and cerebrovascular diseases [7] associated with high cholesterol concentration. This also includes heart stroke, peripheral artery disease, diabetes, and high blood pressure [8]. Therefore, the cholesterol-lowering ability of probiotics and the associated mechanisms for cholesterol removal have received a great attention. However, the exact mechanism of the cholesterol removal by probiotics is not completely understood but several mechanisms have been proposed through in-vivo and in-vitro studies [9]. Probiotic strains such as Lactobacillus fermentum, Limosilactobacillus reuteri, Lacticaseibacillus rhamnosus, and Lactobacillus acidophilus are reported for their cholesterol removal ability. Reports have shown that consumption of these probiotics supplemented food can help in cholesterol reduction by producing bile salt hydrolase (BSH) enzyme and therefore, production of BSH has become a crucial selection criterion for probiotic organisms to determine their cholesterol removal ability. Besides producing BSH, probiotics also possess some inherent ability to scavenge cholesterol [8]. With reference to this, we have also screened out our isolates on the basis of BSH production and assessed their potential for cholesterol removal.
Nowadays, another problem faced by many people is lactose intolerance [10]. Lactose intolerance is not a disease but it is a physiological condition where this disaccharide can cause severe intestinal distress such as abdominal pain, bloating, and flatulence in a person with deficiency or with lower production of the intestinal enzyme β-galactosidase. Lactose intolerance severely limits the use of milk and other dairy products. Moreover, this condition becomes more severe with age and it restricts the intake of calcium-rich dairy foods when it is actually obligatory. Probiotics play a key role by producing β-galactosidase enzyme during fermentation which hydrolyses the lactose to glucose and galactose and thereby improving lactose digestion [11]. Therefore, in the present study, probiotic isolates were assessed for their ability to produce β-galactosidase enzyme.
In human body, a number of metabolic product are generated in which synthesis of superoxide anion radicals, hydrogen peroxide, and hydroxyl radicals have been reported as reactive oxygen species (ROS) which generate oxidative stress. Moreover, nitrogen oxides, herbicides, ozonization, radiation, and some metals are also responsible for inducing oxidative stress. However, many enzymatic (glutathione reductase, glutathione peroxidase, and superoxide dismutase [SOD]) and non-enzymatic (Vitamin E, Vitamin C, thioredoxin, and glutathione) antioxidant defense mechanisms are reported in living systems that reduce oxidative stress by scavenging ROS. Other than these natural defense mechanisms, several synthetic antioxidants are also available such as butylated hydroxyl-anisole and butylated hydroxyl-toluene [12]. In this context, we have analyzed our probiotic isolates for their antioxidant property using four different methods (Reducing power assay [RPA], 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid [ABTS] assay, 2,2-diphenyl-1-picrylhydrazyl [DPPH] assay, and SOD assay).
Another serious metabolic disorder is diabetes mellitus which shows elevated blood sugar level because of insufficient insulin production (Type 1) or insulin resistance (Type 2). A number of anti-diabetic drugs have been developed to combat this disease. However, the commonly used synthetic drugs have their side effects such as flatulence, stomach pain, nausea, headache, heartburn, and diarrhea. Therefore, as an alternative to these common drugs, researchers have focused on the use of probiotics in the treatment of diabetes mellitus and probiotics have shown an incredible role in diabetes treatments due to their ability to inhibit the enzymes α-glucosidase and a-amylase [13]. Hence, in the present study, we have also assessed probiotic isolates for their anti-diabetic activity.
2. MATERIALS AND METHODS
2.1. Chemicals and Media
de Man Rogosa Sharpe (MRS) broth, Nutrient broth, Agar powder, Cholesterol, ABTS, DPPH, ONPG, pNPG, CTAB, KCl, Na2HPO4, Na2CO3, CaCO3, MgSO4, MnSO4, KOH, NaOH, HCL, H2SO4, NaCl, NaHCO3, KI, β-mercaptoethanol, Pyrogallol, Ox-bile, Starch, Acarbose, Ascorbic acid, O-phthalaldehyde reagent, SOD, α-amylase, α-glucosidase, Trypsin and Pepsin were used in this study. DPPH was purchased from Sigma-Aldrich (U.S.A.) and all the other media, chemical and reagents were purchased from Himedia Laboratories Private Ltd. (Mumbai, India) and Loba Chemie Private Ltd. (Mumbai, India).
2.2. Determination of BSH Activity of Potential Probiotic Isolates by Plate Method
Isolation of probiotic microorganisms was carried out from various samples such as fermented idli batter, curd, human milk, lentils, and an infant’s fecal material and a total of 27 isolates were obtained. They were primarily screened for acid and bile tolerance; 12 isolates have shown resistance to low pH and bile. Further, they were proceeded for secondary screening. Among 12 isolates, nine isolates were screened out showing significant viability during gastrointestinal simulation. These nine isolates were further studied for different health beneficial properties. To determine cholesterol removal ability, BSH activity was done using plate assay method [14]. Soft MRS agar was prepared by adding 1.5% agar to MRS broth and then supplemented with bile salt (0.3% w/v, ox bile) and CaCO3 (0.3% w/v). This mixture was autoclaved, poured on MRS plate, and was allowed to solidify. The wells of 3 mm diameter were made on agar plate and overnight grown cultures were added in wells. The plates were incubated at 37ºC for 72 h and observed after each 24 h time interval for clear visible halos around the well indicated the positive BSH activity of the isolates. MRS plates without bile salts were used as the negative control.
2.3. Bacterial Growth and Cholesterol Removal
Probiotic isolates with good BSH activity were selected for cholesterol removal study. For the same, overnight grown cultures of probiotics were inoculated in medium containing cholesterol (200 μg/ml) supplemented with ox bile (0.3% w/v), MgSO4 (0.05% w/v), and MnSO4 (0.02% w/v). This mixture was then incubated at 37°C under shaking (100 rpm) for 24 h. After incubation, the bacterial growth was measured at 600 nm using a UV-VIS spectrophotometer and cholesterol removal was determined according to the method described by Rudel and Morris [15] with some modifications. Briefly, activated cells were centrifuged (5000 g, 4°C, 10 min) and the supernatant was saved for cholesterol estimation. In 1 ml aliquot, 1 ml of KOH (33% w/v) and 2 ml of absolute ethanol were added. The mixture was then vortexed for 1 min and incubated at 37°C for 15 min. After incubation, 2 ml of distilled water and 3 ml of n-Hexane were added. This mixture was again vortexed for 1 min and allowed to settle down. 1 ml from the hexane layer was transferred to another glass tube and evaporated. After evaporation, the residues were immediately dissolved in 2 ml of O-phthalaldehyde reagent. On complete mixing, 0.5 ml of concentrated H2SO4 was added and the mixture was vortexed again for 1 min. After 10 min, the absorbance of the mixture was taken at 550 nm. All experiments were performed in triplicate. The cholesterol concentration was determined from a standard curve prepared using a cholesterol stock solution (1000 μg/ml). The ability of cholesterol removal of probiotics was expressed as the percentage of cholesterol removed at each incubation interval as follows:
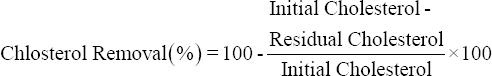 |
2.4. Time Profile for Cholesterol Removal Study
With the observation of bacterial growth and cholesterol utilizing ability, potential probiotic isolate was selected for time profile study. For the same, 24 h grown culture was inoculated into cholesterol containing medium. The concentration of cholesterol was estimated by withdrawing the samples at different time interval such as 0 h, 2 h, 4 h, 6 h, 12 h, and 24 h. The cholesterol concentration was evaluated in triplicate as described earlier.
2.5. Removal of Variable Concentrations of Cholesterol
Cholesterol utilizing ability of probiotic was determined by supplementing different cholesterol concentrations into the medium. Overnight grown culture was inoculated in media having variable cholesterol concentrations ranging from 100 to 500 μg/ml and incubated for 24 h at 37°C. After incubation, the samples were collected and estimated for residual cholesterol content as per the earlier protocol. All the experiments were performed in triplicate.
2.6. Cholesterol Removal by Live and Heat-killed (Dead) Probiotic Cells
The cholesterol removal ability of non-growing (heat-killed) probiotic cells was compared with growing cells. Heat-killed (dead) cells sample was prepared by autoclaving the overnight grown culture at 121°C for 15 min. After that, heat killed dead cells and live growing cells were processed for cholesterol removal study as described earlier. After incubation, cholesterol concentrations of the supernatants were determined. All the experiments were performed in triplicate.
2.7. Removal of Cholesterol from Egg Yolk under Gastrointestinal Stress by Probiotics
Probiotic isolate was examined for its ability to remove cholesterol from cholesterol-rich food source (egg yolk) under gastrointestinal stress condition to mimic the in vivo condition. Egg yolk was added to phosphate buffer (0.2 M, pH 7.0) and then homogenized. This homogenized egg yolk was taken as a sole source of nutrition in the media and inoculated with the overnight grown culture of probiotic isolate. This system was treated with gastric (for 2 h), intestinal (for 4 h), and gastrointestinal (for 6 h) simulation. After each simulation treatment, the samples were collected and the cholesterol content was estimated in triplicate from the supernatant using the same method.
2.8. Assessment of Lactose Utilizing Ability of Probiotic Isolates
Lactose fermentation results in organic acid production [3] and thus lactose utilization ability of probiotics can be determined by observing the color change in the medium as a result of fermentation. Fort the same, 24 h grown probiotic cultures were inoculated in fermentation medium (10 g peptone, 15 g NaCl, 0.018 g phenol red, 5 g lactose in 1 L distilled water, and pH 7.0). These tubes were incubated at 37°C for 24 h and after incubation, the tubes were observed for the change in color from red to yellow indicating acid production on lactose fermentation.
2.8.1. Determination of β-galactosidase activity
Cultures showing good lactose utilizing ability were selected for the determination of β-galactosidase activity using the method describes by Özkan et al. [6] with several modifications. For this purpose, absorbance of activated cultures was taken at 600 nm and then 20 μl of 24 h grown cultures were mixed with 80 μl of permeabilization solution (100 mM Na2HPO4, 20 mM KCL, 2 mM MgSO4, 0.8 μg/ml CTAB, 0.4 μg/ml SDS, and 5.4 μl/ml β-mercaptoethanol) followed by incubation at 37°C for 20–30 min. Further, 600 μl substrate solution (60 mM Na2HPO4, 40 mM NaH2PO4, 1 μg/ml O-nitro-phenyl- β-D-Galactoside (ONPG), 2.7 μl/ml β-mercaptoethanol) was added and the reaction mixture was allowed to incubate at 37°C for 15 min or until the yellow color appears and the reaction completion time period was noted down. After incubation, the reaction was stopped by adding 700 μl of stop solution (1 M Na2CO3). Consequently, absorbance at 420 nm was measured and the β-galactosidase activity was determined using the following formula:
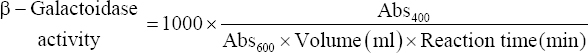 |
2.9. Determination of Antioxidant Activity
2.9.1. RPA assay
RPA was performed as per the method used by Al-Dhabi et al. [12] with some modifications. 100 ml of samples (cell-free supernatant [CFS]) were mixed with 1% K3FeCN6 and incubated at 50°C for 20 min in boiling water bath. The mixture was allowed to cool down and 10% trichloroacetic acid was added. Further, the content was centrifuged and the upper layer was aspirated and mixed with 0.1% FeCl3 solution. Then, the absorbance was taken at 700 nm and the reducing power potential was determined with reference to ascorbic acid standard in terms of ascorbic acid equivalent antioxidant capacity (AEAC, μg/ml).
2.9.2. ABTS assay
Antioxidant activity was also determined by ABTS assay [16]. As per the protocol, 5 ml of ABTS (7 mM) solution and 88 μl of potassium persulphate (140 mM) were mixed vigorously. This mixture was incubated at room temperature for at least 16 h in the dark. Then, this solution was diluted with distilled water until it reaches to optical density 0.7 at 734 nm and this was used as working solution. 100 μl of sample (CFS) was mixed with 3 ml of ABTS working reagent and incubated at 37°C for 10 min after which the absorbance was taken at 734 nm. A control without sample was also measured and the percent inhibition activity was calculated using the following formula:
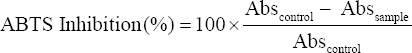 |
2.9.3. DPPH assay
Potential isolates were evaluated for their ability to scavenge DPPH free radicals as suggested by Sui et al. [17] with slight alterations. 50 μl of sample (CFS) was mixed with 3 ml DPPH (0.2 mM) solution and incubated at 37°C for 20 min. Then, the absorbance was measured at 517 nm along with the control (without sample) and the percent inhibition activity was calculated using the following formula:
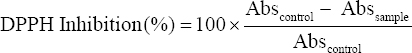 |
2.9.4. SOD activity
Superoxide scavenging activity was also determined for potential isolates [18]. Briefly, 2.35 ml of Tris-buffer (0.1 M) was mixed with 2 ml of distilled water followed by 20 μl of sample (CFS) and 150 μl of pyrogallol solution (4.5 mM in HCL). For the blank tube, same reaction mixture was prepared without adding sample and its absorbance was taken just after mixing the reagent and also after 1 min of mixing. The difference in absorbance between two aliquots indicated the rate of pyrogallol autoxidation. The superoxide radical scavenging activity was calculated as:
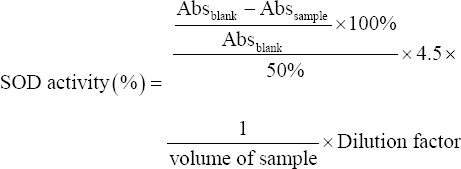 |
2.10. Anti-diabetic Activity of Potential Probiotic Isolates
Pancreatic α-amylase and α-glucosidase are two major enzymes present in digestive system that catalyze the first step in the digestion of starch. Inhibition of these enzymes decreases the digestion and uptake of carbohydrates, thereby curtailing postprandial blood glucose level is regularized in Type II diabetic subjects. Therefore, here in this study, anti-diabetic activity was determined by performing these two tests where acarbose was taken as standard.
2.10.1. Anti-α-amylase activity
Anti-α-amylase activity was determined by the method suggested by Xia et al. [19]. For the same, 100 μl aliquot (CFS) was mixed with 300 μl of sodium phosphate buffer (0.1 M, pH 6.9), then 200 μl of α-amylase (2 U/mg) enzyme was added and the mixture was incubated at 37°C for 10 min. On incubation, 200 μl of starch (1% w/v) was added and enzymatic reaction was allowed for 30 min at 37°C. After completion, the reaction was stopped by adding 200 μl of HCl followed by 200 μl of KI for color development and then the absorbance was taken at 620 nm. A solution mixture without substrate and enzyme was used as blank and without enzyme was used as a control and the inhibition activity was calculated using the following formula:
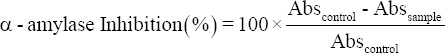 |
2.10.2. Anti-α-glucosidase activity
Anti-α-glucosidase activity of selected probiotic cultures was also determined [20]. Briefly, 100 μl of sample (CFS) was taken and mixed with 400 μl sodium phosphate buffer (0.1 M, pH 6.9). 100 μl of α-glucosidase enzyme (1 U/ml) was added and tubes were incubated at 37°C for 10 min. After this pre-incubation, 200 ml of p-nitro-phenyl a-glucopyranoside (pNPG, 5 mM) was added as a substrate solution and enzymatic reaction was allowed for 30 min at 37°C. After incubation, the reaction was stopped by adding 100 μl of 0.1 M sodium carbonate and then the absorbance was measured at 405 nm. Anti-α-glucosidase activity was determined by measuring the release of p-nitro-phenol from pNPG at 405 nm. A solution mixture without sample was used as a control and without sample and enzyme was used as blank and the inhibition activity was calculated as follows:
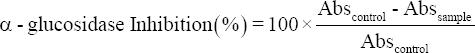 |
2.11. Statistical Analysis
All experimental measurements were repeated independently in triplicate and results are expressed as mean ± standard deviation and they were evaluated by performing the analysis of variance (ANOVA) considering the significance level of P < 0.05. The analysis was performed using MS-Excel.
3. RESULTS AND DISCUSSION
3.1. Determination of BSH Activity of Potential Probiotic Isolates by Plate Method
The BSH enzyme produced by probiotics perhaps deconjugates the bile salts which leads to less cholesterol uptake from the gut [17]. This can be used as an vital marker for selection of unique probiotics having hypocholesterolemic activity [1]. Total nine different probiotic isolates were tested for the production of BSH enzyme on MRS agar plate supplemented with bile salt and calcium carbonate by agar well diffusion method. Among them, isolate D25, B11, B32, M2, and M3 have shown positive BSH activity by showing a clear zone around the well [Figure 1] and the zone diameters are shown in Figure 2. Similarly, Foley et al. [21] have determined BSH activity of L. acidophilus and Lactobacillus gasseri and suggested that BSH enzyme cleave the conjugated glycine or taurine from primary bile acids which may help further in cholesterol removal. In addition, Liang et al. [22] have reported BSH activity of 54 highly hydrophobic probiotic isolates by the plate assay against taurodeoxycholic acid (TDCA) or glycinodeoxycholic acid (GDCA). They found that 15 isolates have shown positive activity for both the substrates, 13 had ability to hydrolyzed TDCA, and only five strains tended to hydrolyze GDCA. Hence, they concluded that different strains might have different substrate specificity of BSH. Moreover, Zhang et al. [9] have determined BSH activity of 10 different Streptococcus thermophilus strains and compared it with standard reference strain where they found that three isolates have higher, three isolates have lower, and four isolates have similar BSH activity to the reference strain.
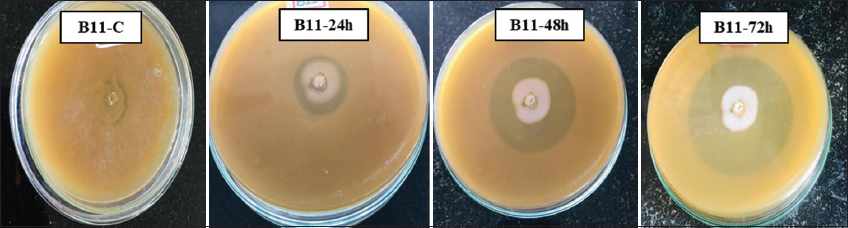 | Figure 1: Bile salt hydrolase activity zone on bile salt supplemented MRS agar plates. [Click here to view] |
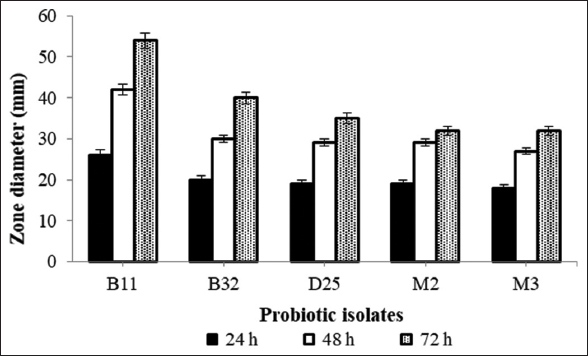 | Figure 2: Bile salt hydrolase activity of probiotic isolates by plate method. [Click here to view] |
3.2. Bacterial Growth and Cholesterol Removal
Total three potential BSH producing probiotic isolates (isolate B11, D25, and B32) were selected for cholesterol removal study. Activated cultures were inoculated into the medium with cholesterol and bile and isolate B11 has shown the highest cholesterol removal efficiency (88 ± 0.2%) with significant growth [Figure 3]. On similar notes, Liu et al. [23] have studied cholesterol removal ability of Lactiplantibacillus plantarum Y15 through both in-vitro and in-vivo studies. They have observed high BSH activity in-vitro. In in-vivo study, they have observed a decrease in total cholesterol and triglyceride level in serum and liver while level of cholesterol and bile salts was increased in feces. Likewise, Singhal et al. [24] have isolated Enterobacter faecium which was able to assimilate >50% of cholesterol from the medium. This percent assimilation could be improved with addition of bile salts in the medium as E. faecium LR13 has shown 98% and 76% assimilation with and without bile salts, respectively. In addition, El-Zahar et al. [25] have supplemented camel and cow’s milk with Bifidobacterium longum and observed that it significantly improved the body weight, blood lipid profile, serum proteins, and liver and kidney markers in mice fed with high fat diet. Moreover, Pereira et al. [26] have studied cholesterol removal from MRS medium containing cholesterol along with ox-bile. They concluded that the amount of bile salt present in the medium has affected both the growth rate of bacteria as well as its cholesterol removal ability. As the amount of ox-bile was increased, a significant decrease was observed in the growth rate and so as in cholesterol removal. At lower bile concentration (<0.5%), cholesterol reduction remained constant, and it increased till 2% cholesterol concentration and then with further rise in concentration, no significant removal was observed.
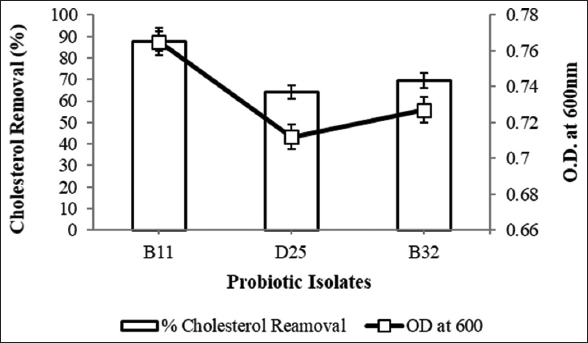 | Figure 3: Bacterial growth and cholesterol removal efficiency. [Click here to view] |
3.3. Time Profile for Cholesterol Removal Study
Time profile of cholesterol removal was carried out with isolate B11. The significant finding of this study is that this culture was able to utilize more than 60% of supplemented cholesterol within 6 h and the highest cholesterol removal was observed 88 ± 0.17% after 24 h as shown in Figure 4. Similar kind of results has been reported by Kimoto et al. [27] where maximum cholesterol removal by isolate N7 was achieved within 12–18 h of incubation which corresponds to the exponential growth of the culture. Comparable statement was also given by Shivangi et al. [18] that cholesterol removal depends on bacterial cellular state and growth phase (log, stationary, and decline phase). Their isolate Bacillus tequilensis showed maximum cholesterol removal (64.41%) in log phase of growth.
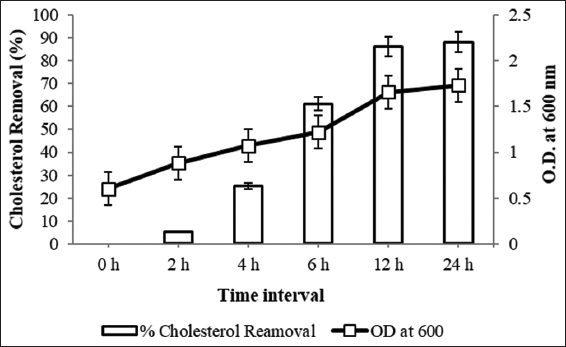 | Figure 4: Time profile study for cholesterol removal. [Click here to view] |
3.4. Cholesterol Removal with Different Cholesterol Concentration
Isolate B11 has shown significant removal within 24 h and therefore to determine cholesterol removal efficacy of this culture, variable concentrations of cholesterol (100, 200, 300, 400, and 500 μg/ml) were taken. After 24 h of incubation, cholesterol removal was determined and linear decrease was observed with low to high concentrations. The observations indicated lower removal efficiency with increase in cholesterol concentration into the media [Figure 5]. Isolate B11 showed 92 ± 0.11% removal with the lowest concentration (100 μg/ml) which was decreased to 72 ± 0.55% with 500 μg/ml cholesterol. In contrast, Majeed et al. [28] observed regardless of minimum (25 μg/ml) or maximum (200 μg/ml) concentrations of cholesterol, Bacillus coagulans was able to remove nearly 50% of total cholesterol from the medium.
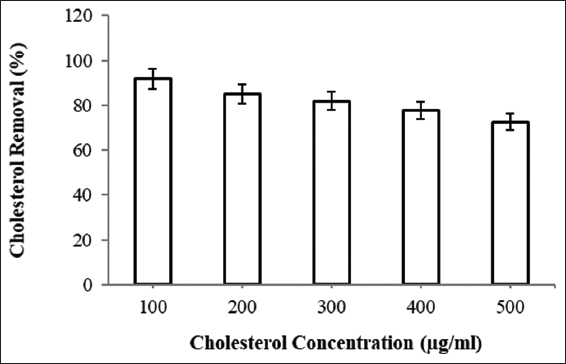 | Figure 5: Cholesterol removal at variable cholesterol concentration. [Click here to view] |
3.5. Cholesterol Removal by Live and Heat-killed (Dead) Probiotic Cells
The actual mechanism of cholesterol removal is still unknown; and therefore, this study was also carried out with live and dead biomass to determine whether the dead cells can assimilate the cholesterol or not. Two different concentrations 200 and 500 μg/ml were taken under study and cholesterol removal was observed 86 ± 0.04% and 73 ± 0.13% with live cells and 8 ± 0.2% and 9 ± 0.04% with dead biomass, respectively. These observations indicated that dead biomass has no significant effect on cholesterol removal. The capability of dead cells to confer health benefits has attracted many food manufactures due to certain advantages such as longer shelf-life, better handling, easy transportation and storage, and possibility of addition of non-bacterial biologically active metabolite. However, simultaneously, inactivated or killed cells possess negligible functional properties and therefore do not extend any health benefits in diseased conditions [29]. In this reference, Miremadi et al. [30] reported significant difference in cholesterol removal by growing (34–65%), resting (29–56%), and dead (9–37%) cells of L. acidophilus and B. longum. Likewise, Anila et al. [31] have described higher cholesterol removal (47.70 μg/ml) by live cells of Lacticaseibacillus casei PLA5 which was only 4.74 μg/ml by dead cells indicating the attachment of cholesterol on the cell membrane of live bacterial cells [32].
3.6. Removal of Cholesterol from Egg Yolk under Gastro-intestinal Stress by Probiotics
Cholesterol removal in a synthetic medium was studied with many different parameters so the isolate B11 was assessed for its removal efficiency from a cholesterol rich food (egg yolk). To mimic in vivo condition, simulation treatment was also given and the cholesterol removal was observed 15 ± 0.12% after 2 h of gastric (GJ), 15 ± 0.24 % after 4 h of intestinal (IJ), and 45 ± 0.09 % after 6 h of gastrointestinal (G + I) simulation [Figure 6]. When the treatment was continued for 24 h, it resulted in 52 ± 0.02% removal. In a similar way, Mikulski et al. [33] have observed 12.33% cholesterol removal from egg yolk by Pediococcus acidilactici. With a step ahead, Lokapirnasari et al. [34] aimed to study the combitorial effect of Bifidobacterium spp. and L. acidophilus on egg yolk cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) content. With supplementation of equal amount (0.5% each) of both the probiotics, they observed lower cholesterol content (155.86 mg/dl), lower LDL content (48.46 mg/dl), and higher HDL (50.00 mg/dl) which were earlier observed 224.02 mg/dl, 58.63 mg/dl, and 41.59 mg/dl, respectively, suggesting probiotics as a significant diet supplement. Researchers have also studied cholesterol removal potential of probiotics with other cholesterol rich foods than egg yolk. With reference to this, Liu et al. [35] have determined cholesterol removal ability of L. plantarum and Enterococcus faecalis from egg yolk as well as skimmed milk. They observed 58.15% and 38.45% of cholesterol removal from egg yolk and 22.35% and 11.22% removal from skimmed milk by L. plantarum and E. faecalis, respectively. Their removal pattern is also similar to that of observed in our study.
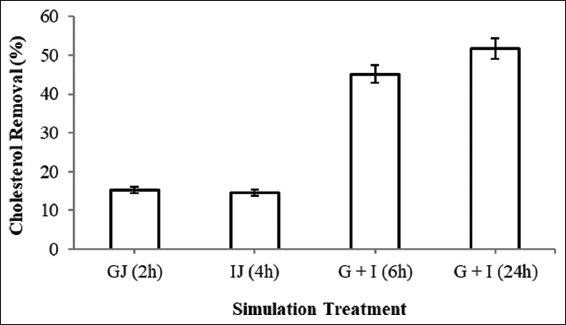 | Figure 6: Cholesterol removal from egg yolk with simulation treatment. (GJ - Gastric juice, IJ - Intestinal juice, G + I - Gastrointestinal simulation). [Click here to view] |
3.7. Determination of Lactose Utilizing Ability
Probiotic organisms have been proved very useful for lactose intolerant people as they can easily utilize lactose. Total nine potential isolates were screened for their lactose utilizing ability by tube method where phenol red was used as an indicator. Organism with lactose utilizing ability produces organic acids on fermentation of lactose that leads to yellow color production in the medium. In this study, total six isolates (Isolate B32, D25, F, M1, M2, and M3) have shown positive lactose utilizing ability. This lactose utilizing ability is considered as screening criteria for the selection of potential probiotic [36] and Zeng et al. [37] have studied fermentation patterns of three different L. plantarum strains (GS083, GS086, and GS090) and observed all of them were able to utilize lactose as a carbon source.
3.8. Determination of β-galactosidas eActivity
Many children and adults are unable to digest lactose due to insufficient production of β-galactosidase enzyme and suffer from lactose intolerance which is a common digestive problem when person consume milk or any other dairy products [38]. Probiotic organisms have been found beneficial in improving these symptoms through the production of β-galactosidase [6]. In this study, all lactose utilizing probiotic isolates were screened for β-galactosidase activity by ONPG method. From the six potential lactose utilizing isolates, isolate D25 has shown maximum activity of 226 ± 0.30 Miller units followed by isolate B32 (190 ± 0.13) and M2 (110 ± 0.15) as shown in Figure 7. Similarly, Son et al. [39] have studied β-galactosidase activity of L. plantarum Ln4 isolated from Kimchi that significantly showed 3320.99 Miller units activity. In addition, Chanalia et al. [40] have reported β-galactosidase activity of P. acidilactici to be 65.08 ± 0.9 Miller units which was actually 22 times higher than earlier reports. In addition, Zhao et al. [41] have determined β-galactosidase activity of Lactobacillus bulgaricus with the use of whey-based medium and observed two-fold higher (2034 U/L) activity than the activity observed with the traditional MRS medium. However, probiotic strain Lactobacillus lactis Gh1 was not able to produce β-galactosidase enzyme but they suggested that absence of this enzyme would not be a disadvantage since permeability of cells is necessary for efficient lactose hydrolysis in the small intestine [42].
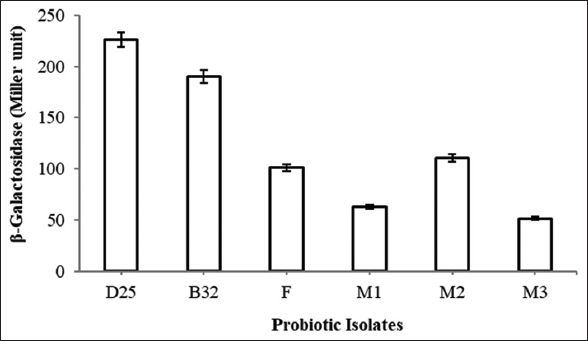 | Figure 7: β-Galactosidase activity of probiotic isolates. [Click here to view] |
3.9. Determination of Antioxidant Activity
In the present study, antioxidant activity of potential isolates was determined using four different methods such as RPA, ABTS, DPPH, and SOD. RPA was determined in terms of ascorbic (AEAC, μg/ml) and isolates have shown significant reduction ability except isolate F [Figure 8a]. Isolate M3 (1074 ± 0.32 μg/ml) showed the highest AEAC followed by isolate B32 (953 ± 0.37 μg/ml) and D25 (905 ± 0.26 μg/ml). When ABTS assay was performed [Figure 8b], isolate D25 (72 ± 0.04%) showed maximum ABTS scavenging ability followed by isolate B11 (69 ± 0.17%) and B32 (66 ± 0.46%). DPPH assay was also performed where isolate D25 (71 ± 0.22%) has again shown maximum DPPH scavenging activity followed by isolate B11 (64 ± 0.26%) and isolate M2 with 61 ± 014% activity [Figure 8c]. Moreover, in case of SOD activity [Figure 8d], isolate D25 and B11 have shown 25 ± 0.06% and 23 ± 0.33% activity, respectively. Similarly, Cao et al. [16] studied L. plantarum ST with significant ABTS (47%) and DPPH (59%) scavenging activity. Antioxidant activity with CFS, cell free extract and intact cells of L. plantarum and L. rhamnosus was determined by DPPH method and highest antioxidant activity with CFS followed by cell free extract and intact cells was observed [43]. In addition, Romero-Luna et al. [44] have reported 1336.72 ± 345.05 Troloxeq ABTS scavenging activity from the CFS of L. paracasei CT12. Furthermore, tremendous DPPH scavenging activity was observed from the CFS of Weissella confusa MD1 (69.15 ± 2.73%) and Weissella cibaria MD2 (74.34 ± 1.4%) [45]. In addition, 50% reducing power potential of L. plantarum RJF4 by RPA was also reported [46]. In addition, Kostelac et al. [47] determined SOD activity of their isolate L. plantarum M2 (0.02 ± 0.002 U/ml) and L. plantarum KO9 (0.05 ± 0.009 U/ml).
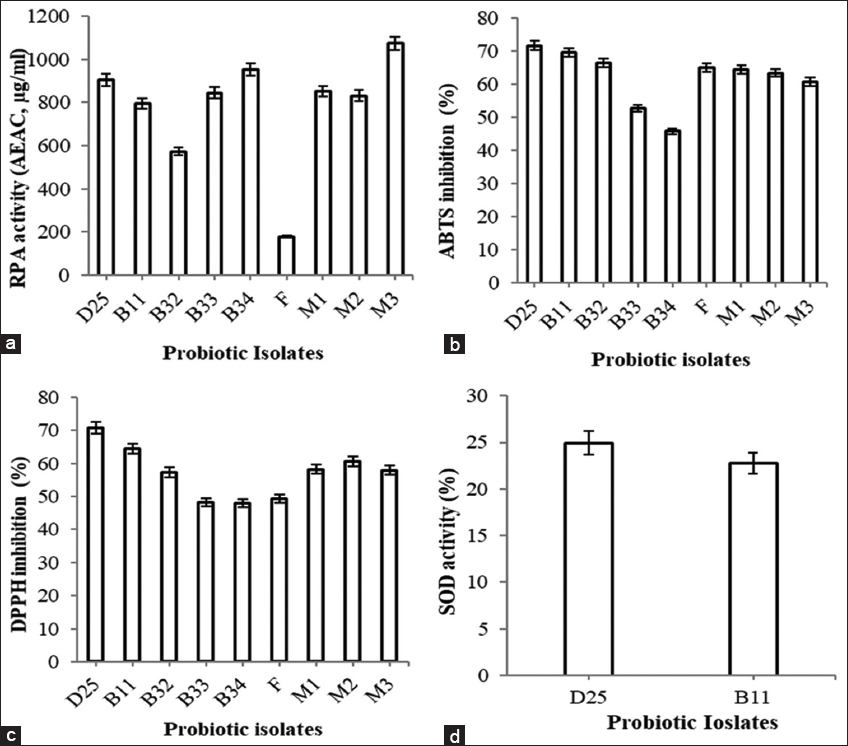 | Figure 8: Antioxidant activity of probiotic isolates (a) reducing power activity, (b) 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid inhibition activity, (c) 2,2-diphenyl-1-picrylhydrazyl inhibition activity, and (d) superoxide dismutase activity. [Click here to view] |
3.10. Anti-α-amylase and Anti-α-glucosidase Activity
Amylase is a first enzyme to start digestion and it will convert starch to glucose molecule. Similarly, α-glucosidase enzyme also helps in digestion by breaking down starch and other disaccharides into glucose molecules. Inhibition of these two enzymes by probiotic cells leads to low serum glucose level and proves them as potent anti-diabetic agents. This activity was performed with two potential isolates, that is, isolate D25 and B11 [Figure 9]. None of them have shown significant inhibitory effect on α-amylase as the inhibition observed was 11 ± 0.24% and 10 ± 0.20%, respectively. This might have happened as these cultures are potential amylase producers and therefore they do not possess any inhibitory effect on this enzyme. However, 89 ± 0.01% and 98 ± 0.30% α-glucosidase inhibition was observed with isolate D25 and B11, respectively. Similarly, potent inhibitory activity of α-glucosidase (91.7%) by L. plantarum C70 [48]. Likewise, Zeng et al. [13] have assessed α-glucosidase inhibition activity of seven different strains of L. plantarum and L. brevis and observed inhibitory activities ranged from 18.4% to 34.9% with the highest value for L. plantarum ZF06-3. Our result with α-glucosidase enzyme was considerably significant than the earlier reported observations but our α-amylase inhibition is not significant. Similar kind of observation was also reported by Shin et al. [49]. They observed no amylase inhibition activity with their 17 probiotic isolates and have suggested that high amylase activity is a fundamental property of probiotics for starch hydrolysis and this might be a reason behind low inhibitory activity.
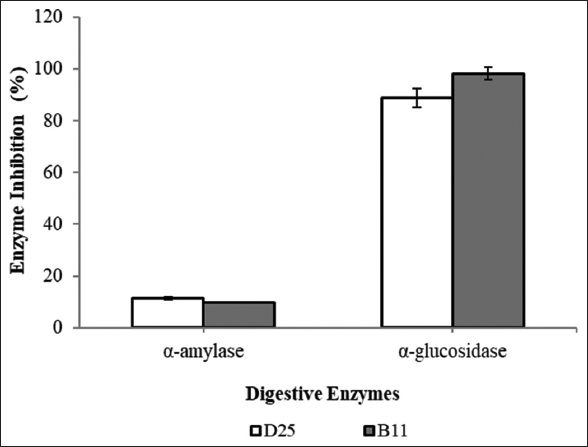 | Figure 9: Anti-diabetic activity of potential probiotic isolates. [Click here to view] |
As isolate D25 and B11 have shown very significant results in terms of various health beneficial properties, they were further identified by 16S rRNA sequencing method. Isolate D25 and B11 were identified as Lactiplantibacillus plantarum with accession number MW362778 and Pediococcus pentosaceus with accession number MW362744, respectively.
4. CONCLUSION
The results of the present study indicate that all the nine isolates taken under study have shown significant results in terms of cholesterol removal, β-galactosidase production, antioxidant property, and anti-diabetic activity. Among them, isolate B11 and D25 have shown highly potent results for all the properties. Now a day, many synthetic drugs are available in market to solve such health issues but they all have some side effects on human and animal health. Hence, as an alternative to these synthetic drugs, probiotic organisms can be used which can give multifarious health benefits. Moreover, these probiotics can be consumed separately as well as they can be added in the preparation of functional foods, can be utilized as an animal feed supplement, and can be utilized in cosmetic industry and also for some clinical purposes.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
6. FUNDING
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All data generated or analysed during this study are included in this article.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Bhat B, Bajaj BK. Multifarious cholesterol lowering potential of lactic acid bacteria equipped with desired probiotic functional attributes. 3 Biotech 2020;10:200. [CrossRef]
2. Sharma A, Lavania M, Singh R, Lal B. Identification and probiotic potential of lactic acid bacteria from camel milk. Saudi J Biol Sci 2021;28:1622-32. [CrossRef]
3. Pundir RK, Rana S, Kashyap N and Kaur A. Probiotic potential of lactic acid bacteria isolated from food samples:An in vitro study. J Appl Pharm Sci 2013;3:85-93.
4. Singh V, Ganger S, Patil S. Characterization of Lactobacillus brevis with potential probiotic properties and biofilm inhibition against Pseudomonas aeruginosa. Proc 2020;66:1-7. [CrossRef]
5. Zhang J, Zhang H, Wang L, Zhang K, Qiu Z, Zhang K, et al. The safety and potential probiotic properties analysis of Streptococcus alactolyticus strain FGM isolated from the chicken cecum. Ann Microbiol 2021;71:1-140. [CrossRef]
6. Özkan ER, Demirci T, Öztürk H?, Ak?n N. Screening Lactobacillus strains from artisanal Turkish goatskin casing Tulum cheeses produced by nomads via molecular and in vitro probiotic characteristics. J Sci Food Agric 2021;101:2799-808. [CrossRef]
7. Nguyen TD, Kang JH, Lee MS. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int J Food Microbiol 2007;113:358-61. [CrossRef]
8. Kathade S, Aswani M, Anand PK, and Nirichan B. Probiotic characterization and cholesterol assimilation ability of Pichia kudriavzevii isolated from the gut of the edible freshwater snail 'Pila globosa'. Egypt J Aquat Biol Fish 2020;24:23-39. [CrossRef]
9. Zhang J, Liu M, Xu J, Qi Y, Zhao N, Fan M. First insight into the probiotic properties of ten Streptococcus thermophilus strains based on in vitro conditions. Curr Microbiol 2020;77:343-52. [CrossRef]
10. Kun S, Rezessy-SzabóJM, Nguyen QD, Hoschke A. Changes of microbial population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Process Biochem 2008;43:816-21. [CrossRef]
11. Goldin BR. Health benefits of probiotics. Braz J Nutr 1998;80:203-7. [CrossRef]
12. Al-Dhabi NA, Valan Arasu M, Vijayaraghavan P, Esmail GA, Duraipandiyan V, Kim YO, et al. Probiotic and antioxidant potential of Lactobacillus reuteri lr12 and Lactobacillus lactis ll10 isolated from pineapple puree and quality analysis of pineapple-flavored goat milk yoghurt during storage. Microorganisms 2020;8:1-15. [CrossRef]
13. Zeng Z, Luo J, Zuo F, Zhang Y, Ma H, Chen S. Screening for potential novel probiotic Lactobacillus strains based on high dipeptidyl peptidase IV and a-glucosidase inhibitory activity. J Funct Foods 2016;20:486-95. [CrossRef]
14. Sirilun S, Chaiyasut C, Kantachote D, Luxananil P. Characterisation of non human origin probiotic Lactobacillus plantarum with cholesterol-lowering property. Afr J Microbiol Res 2010;4:994-1000.
15. Rudel LL, Morris MD. Determination of cholesterol using o phthalaldehyde. J Lipid Res 1973;14:364-6. [CrossRef]
16. Cao Z, Pan H, Li S, Shi C, Wang S, Wang F, et al. In vitro evaluation of probiotic potential of lactic acid bacteria isolated from Yunnan De'ang pickled tea. Probiotics Antimicrob Proteins 2018;11:103-12. [CrossRef]
17. Sui Y, Liu J, Liu Y, Wang Y, Xiao Y, Gao B, et al. In vitro probiotic characterization of Lactobacillus strains from fermented tangerine vinegar and their cholesterol degradation activity. Food Biosci 2021;39:100843. [CrossRef]
18. Shivangi S, Devi PB, Ragul K, Shetty PH. Probiotic potential of Bacillus strains isolated from an acidic fermented food idli. Probiotics Antimicrob Proteins 2020;12:1502-13. [CrossRef]
19. Xia Y, Qin S, Shen Y. Probiotic potential of Weissella strains isolated from horse feces. Microb Pathog 2019;132:117-23. [CrossRef]
20. Ayyash M, Olaimat A, Al-Nabulsi A, Liu SQ. Bioactive properties of novel probiotic Lactococcus lactis fermented camel sausages:Cytotoxicity, angiotensin converting enzyme inhibition, antioxidant capacity, and antidiabetic activity. Food Sci Anim Resour 2020;40:155-71. [CrossRef]
21. Foley MH, O'Flaherty S, Allen G, Rivera AJ, Stewart AK, Barrangou R, et al. Lactobacillus bile salt hydrolase substrate specificity governs bacterial fitness and host colonization. Proc Natl Acad Sci U S A 2021;118:e2017709118. [CrossRef]
22. Liang X, Zhang Z, Yi H, Liu T, Li R, Yu Z, et al. Study on intestinal survival and cholesterol metabolism of probiotics. LWT Food Sci Technol 2020;124:109-32. [CrossRef]
23. Liu Y, Zheng S, Cui J, Guo T, Zhang J. Effect of bile salt hydrolase-active Lactobacillus plantarum Y15 on high cholesterol diet induced hypercholesterolemic mice. CyTA J Food 2021;19:408-17. [CrossRef]
24. Singhal N, Maurya AK, Mohanty S, Kumar M, Virdi JS. Evaluation of bile salt hydrolases, cholesterol-lowering capabilities, and probiotic potential of Enterococcus faecium isolated from rhizosphere. Front Microbiol 2019;10:1567. [CrossRef]
25. El-Zahar KM, Hassan MF, Al-Qaba SF. Protective effect of fermented camel milk containing Bifidobacterium longum BB536 on blood lipid profile in hypercholesterolemic rats. J Nutr Metab 2021;2021:1557945. [CrossRef]
26. Pereira DI, McCartney AL, Gibson GR. An in vitro study of the probiotic potential of a bile-salt-hydrolyzing Lactobacillus fermentum strain, and determination of its cholesterol-lowering properties. Appl Environ Microbiol 2003;69:4743-52. [CrossRef]
27. Kimoto H, Ohmomo S, Okamoto T. Cholesterol removal from media by lactococci. J Dairy Sci 2002;85:3182-8. [CrossRef]
28. Majeed M, Majeed S, Nagabhushanam K, Arumugam S, Beede K, Ali F. Evaluation of the in vitro cholesterol-lowering activity of the probiotic strain Bacillus coagulans MTCC 5856. Int J Food Sci Technol 2019;54:212-20. [CrossRef]
29. Brouwer AM, Mosack KE. Whether viable and dead probiotic are equally efficacious?Nutr Food Sci 2015;45:39-53. [CrossRef]
30. Miremadi F, Ayyash M, Sherkat F, Stojanovska L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic Lactobacilli and Bifidobacteria. J Funct Foods 2014;9:295-305. [CrossRef]
31. Anila K, Kunzes A, Bhalla T. In vitro cholesterol assimilation and functional enzymatic activities of putative probiotic Lactobacillus sp. isolated from fermented foods/beverages of North West India. J Nutr Food Sci 2016;06:1-5. [CrossRef]
32. Le B, Yang SH. Identification of a novel potential probiotic Lactobacillus plantarum FB003 isolated from salted-fermented shrimp and its effect on cholesterol absorption by regulation of NPC1L1 and PPARa. Probiotics Antimicrob Proteins 2019;11:785-93. [CrossRef]
33. Mikulski D, Jankowski J, Mikulska M, Demey V. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on productive performance, egg quality, and body composition in laying hens fed diets varying in energy density. Poult Sci 2020;99:2275-85. [CrossRef]
34. Lokapirnasari WP, Sahidu AM, Maslachah L, Yulianto AB, Najwan R. The effect of combination Bifidobacterium sp and Lactobacillus acidophilus probiotic on egg yolk cholesterol, HDL, and LDL. IOP Conf Ser Earth Environ Sci 2020;441:1-4. [CrossRef]
35. Liu H, Yang C, Jing Y, Li Z, Zhong W, Li G. Ability of lactic acid bacteria isolated from mink to remove cholesterol:In vitro and in vivo studies. Can J Microbiol 2013;59:563-9. [CrossRef]
36. Devi SM, Halami PM. Genetic variation of pln loci among probiotic Lactobacillus plantarum group strains with antioxidant and cholesterol-lowering ability. Probiotics Antimicrob Proteins 2019;11:11-22. [CrossRef]
37. Zeng Y, Li Y, Wu Q, Zhang J, Xie X, Ding Y, et al. Evaluation of the antibacterial activity and probiotic potential of Lactobacillus plantarum isolated from Chinese homemade pickles. Can J Infect Dis Med Microbiol 2020;2020:1-11. [CrossRef]
38. Gomes TA, Fontana E, Zielinski AA, Nogueira A, Spier MR. Optimizing the growth-associated b-galactosidase production by probiotic Lactobacillus reuteri B-14171:Experimental design, culture medium volume increase, and cell growth modeling. Sci Plena 2021;17:1-19. [CrossRef]
39. Son SH, Jeon HL, Jeon E, Lee NK, Park YS, Kang DK, et al. Potential probiotic Lactobacillus plantarum Ln4 from kimchi:Evaluation of b-galactosidase and antioxidant activities. LWT Food Sci Technol 2017;85:181-6. [CrossRef]
40. Chanalia P, Gandhi D, Attri P, Dhanda S. Purification and characterization of b-galactosidase from probiotic Pediococcus acidilactici and its use in milk lactose hydrolysis and galactooligosaccharide synthesis. Bioorg Chem 2018;77:176-89. [CrossRef]
41. Zhao R, Duan F, Yang J, Xiao M, Lu L. Integrated utilization of dairy whey in probiotic b-galactosidase production and enzymatic synthesis of galacto-oligosaccharides. Catalysts 2021;11:1-12. [CrossRef]
42. Jawan R, Abbasiliasi S, Mustafa S, Kapri MR, Halim M, Ariff AB. In vitro evaluation of potential probiotic strain Lactococcus lactis Gh1 and its bacteriocin-like inhibitory substances for potential use in the food industry. Probiotics Antimicrob Proteins 2021;13:422-40. [CrossRef]
43. Bhushan B, Sakhare SM, Narayan KS, Kumari M, Mishra V, Dicks LM. Characterization of riboflavin-producing strains of Lactobacillus plantarum as potential probiotic candidate through in vitro assessment and principal component analysis. Probiotics Antimicrob Proteins 2021;13:453-67. [CrossRef]
44. Romero-Luna HE, Peredo-Lovillo A, Hernández-Mendoza A, Hernández-Sánchez H, Cauich-Sánchez PI, Ribas-Aparicio RM, et al. Probiotic potential of Lactobacillus paracasei CT12 isolated from water kefir grains (Tibicos). Curr Microbiol 2020;77:2584-92. [CrossRef]
45. Lakra AK, Domdi L, Hanjon G, Tilwani YM, Arul V. Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT Food Sci Technol 2020;125:1-9. [CrossRef]
46. Dilna SV, Surya H, Aswathy R, Varsha K, Sakthikumar D, Pandey A, et al. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT Food Sci Technol 2015;64:1179-86. [CrossRef]
47. Kostelac D, Geric M, Gajski G, Markov K, Domijan A, Canak I, et al. Lactic acid bacteria isolated from equid milk and their extracellular metabolites show great probiotic properties and anti-inflammatory potential. Int Dairy J 2021;112:1-8. [CrossRef]
48. Ayyash M, Abu-Jdayil B, Itsaranuwat P, Galiwango E, Tamiello-Rosa C, Abdullah H, et al. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int J Biol Macromol 2020;144:938-46. [CrossRef]
49. Shin MS, Han SK, Ji AR, Kim KS, Lee WK. Isolation and characterization of bacteriocin-producing bacteria from the gastrointestinal tract of broiler chickens for probiotic use. J Appl Microbiol 2008;105:2203-12. [CrossRef]