1. INTRODUCTION
Oxygen scavengers or absorbers are regularly used in product safety and are helpful in anaerobic experiments. In general, oxygen scavengers inhibit the growth of living organism, including photosynthetic organisms such as algae once if it crosses the critical level. However, it needs for certain experiments such as hydrogen production from algal cells through bypassing the Calvin cycle in photosynthetic process under in vitro conditions [1]. The inclusion of oxygen scavengers in the medium (generally tris-acetate-phosphate [TAP]) inhibits the photosynthetic O2 in algal cultures, which, in turn, lead to hydrogen production through activation of hydrogenase [2]. It is a well-known fact that hydrogenase is oxygen-sensitive, which plays an important role in hydrogen production in algal species. Chemically, different types of oxygen scavengers are used for removing the oxygen such as sodium sulfite (Na2SO3), sodium bisulfite (NaHSO3), sodium dithionite or sodium hydrosulfite (Na2S2O4), sodium metabisulfite (Na2S2O5), and ammonium sulfite [(NH4)2SO3] [3]. Rapid screening of different photosynthetic algae using various oxygen scavengers is one of the best ways to find out the candidate species, biomass content, status of anaerobic condition, and level of hydrogen production [3,4]. Several works on algae were carried out using various oxygen scavengers for anaerobic condition in turn hydrogen production in Chlamydomonas reinhardtii, Chlorella vulgaris, Chlorococcum minutum, and Scenedesmus [3,5,6]. Among all the scavengers, sodium bisulfite was tested frequently with algal samples for growth and H2 production [7].
Algae belong to primitive group of plants which divided into unicellular (microalgae) and multicellular (macroalgae) based on their size [8]. Algae, further, classified into terrestrial and aquatic species based on the habitat and aquatic (both marine and fresh water) species are available in bulk number. Furthermore, based on pigment accumulation, algae are divided into green, blue-green, red, and brown algae [9]. Microalgae own a simple life cycle and have short harvesting cycle which is an advantage to choose these species as biofuel resource [10]. Specifically green and blue-green algae are popular for biofuel production due to their potential photosynthetic pathway [11-13]. Apart from energy sector, algae are a crucial staple for food, fodder, pharmaceutical, nutraceutical, and fertilizer industries. Some of the algae possess the significant quantities of vitamins, proteins, minerals, and lipids [14]. The nutrient content may vary from species to species which, in turn, depends on location, season, temperature, etc. However, certain countries neglecting algal species, and hence, they became orphans without use [15].
The quantity and quality of algal biomass indicates its growth and development. In general, unicellular microalgae are simple forms and chlorophyll is their primary photosynthetic pigment [16]. Hence, chlorophyll accumulation in algal species is an important step for their growth. In extent, research on enhancement of biomass production is a continuous process for algal species due to their enormous benefits [17,18]. In general, estimation of yield by means of biomass content is a regular process. Evaluation of the chlorophyll a, chlorophyll b, and total chlorophylls is one among the simplest ways to estimate the biomass of an organism [19]. Costache et al. [20] mentioned that chlorophylls are abundant in nature which provides green color to algae and have efficient light harvesting role in the process of photosynthesis. Considerable yield of any algal species is totally dependent on the growth and initial biomass accumulation. The biochemical processes involved in the conversion of biomass into biohydrogen were well established using model algal species such as C. reinhardtii and C. vulgaris in the recent past with the aid of oxygen scavengers [3,21]. Asterarcys quadricellulare is a fresh water green alga which belong to class Chlorophyceae [22].
In the current investigation, four oxygen scavengers, that is, sodium sulfite, sodium bisulfite, sodium dithionite, and sodium metabisulfite were tested in A. quadricellulare for its growth and accumulation of biomass which is useful for anaerobic and hydrogen production experiments even in other related algal species. To the best of our knowledge, this is the first report on the effects of oxygen scavengers in growth and biomass accumulation in A. quadricellulare.
2. MATERIALS AND METHODS
A. quadricellulare was a collection from the Department of Center for Advanced Study in Botany, Madras University, Chennai, India. Later, collected algal sample was stored as conventional glycerol stocks in −80ºC and some of the cultures were maintained in agar plates and agar tubes for regular experiments. Before the preparation of various algal media for in vitro screening, all the washed glassware (Borosil, India) was desiccated in an oven (Kemi, K04.3, Ernakulam, India) for further usage. In the current work, TAP medium was used for algal cultures along with inclusion of oxygen scavengers, that is, Na2SO3 (0.0, 0.2, 0.8, 3.2, and 12.8mM), NaHSO3 (0.0, 0.4, 0.8, 1.6, and 3.2 mM), Na2S2O4 (0.0, 0.16, 0.32, 0.40, and 0.80 mM), and Na2S2O5 (0.0, 0.04, 0.08, 0.16, 0.20, and 0.40 mM) independently. Figure 1 illustrates the oxygen scavengers used for the current work. The pH of all the media was adjusted to 7.0 ± 01 with standard pH meter (Elico limited, India) and all the experiments were carried out in 30 mL serum vials which consist 5 mL of medium. It is mandatory to maintain empty space (minimum ¾) in the serum vial to allow algal growth due to aeration facility [3]. Moreover, optimal growth of algae may take around 2–4 days depending on the genus or species or strain in the medium. All the 30 mL serum vials with medium were autoclaved using an autoclave (INLAB Equipment, Madras, India) at 121°C and 103421.36 Pa for 20 min. Further, autoclaved media were cooled down and later moved to laminar air flow chamber for algal inoculation (Hi-Tech products, No. 14, Chennai, India). All the inoculated vials were kept in the orbital shaker (RemiElektrotechnik Limited, Vasai, India) at 140 rpm under light conditions and cultures were grown at 25±1°C in continuous light (107.02 cd) conditions.
 | Figure 1: Oxygen scavengers used for Asterarcys quadricellulare in vitro cultures. [Click here to view] |
In the present work, estimation of chlorophyll content was carried out using modified Arnon’s [23] and Varaprasad et al. [24] methods with 2–3 days old cultures. One gram of fresh algal material of both controls and treated samples were ground with a mortar and pestle using 80% acetone. The homogenate was centrifuged at 5000 rpm for 5 min and the supernatant was saved and the residue was re-extracted with 80% acetone up to the formation of pale yellow residue. Using the supernatant absorbance, values were read at 645 and 663 nm in a ultraviolet (UV) visible Spectrophotometer (Shimadzu UV-1800, India). Total chlorophyll content was estimated using standard Arnon formula and expressed in mg/L fresh weight basis. Three replicates were used in each set and all the experiments were conducted thrice. All the statistical analysis was performed using excel program in personal computer.
3. RESULTS AND DISCUSSION
An effort has been made to understand the effects of Na2SO3, NaHSO3, Na2S2O4, and Na2S2O5 on growth and biomass accumulation in green alga A. quadricellulare. The basic aim of the experiment is to estimate the hydrogen production rate in A. quadricellulare through activation of hydrogenase which, in turn, depends on anaerobic condition. Hence, in vitro screening was carried using four various oxygen scavengers and biomass was estimated using 2–3 days old cultures.
3.1. Effect of Na2SO3 on Growth and Chlorophyll Accumulation in A. quadricellulare
Cultures were initiated in TAP medium with different concentrations of sodium sulfite such as 0.2 mM, 0.8 mM, 3.2 mM, and 12.8 mM, along with control. The inclusion of sodium sulfite with various concentrations in TAP medium promotes the growth and chlorophyll content in A. quadricellulare at optimal concentrations. Optimal growth and high total chlorophyll content (63.23 mg/L) was observed in A. quadricellulare at 3.2 mM of sodium sulfite [Figure 2]. In contrast, high concentration of sodium sulfite reduces the growth and chlorophyll content. Overall A. quadricellulare cultures exhibited resistance at certain extent with this scavenger. Significantly, very few reports are available on the effect of sodium sulfite in algal culture and recently our laboratory established oxygen scavenging capacity of sodium sulfite using green alga C. minutum [3]. Sodium sulfite possesses high electron negativity and also sulfur and oxygen ratio was more when compared to other scavengers utilized in the current investigation [25]. The severe oxygen removing capacity of sodium sulfite is one among the explanations for necrobiosis or death [26].
3.2. Effect of NaHSO3 on Growth and Chlorophyll Accumulation in A. quadricellulare
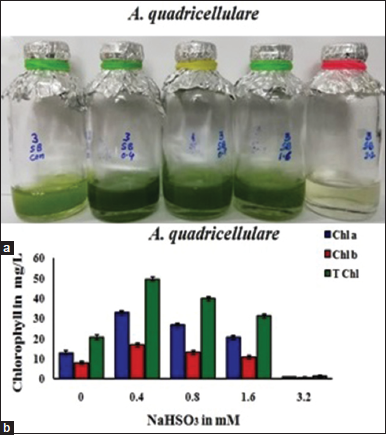
Various concentrations of sodium bisulfite, that is, 0.0 mM, 0.4 mM, 0.8 mM, 1.6 mM, and 3.2 mM were used in TAP medium to know the growth pattern and chlorophyll accumulation in this alga [Figure 3]. Better growth and high total chlorophyll content (49.38 mg/L) was noticed in A. quadricellulare at 0.4 mM of sodium bisulfite. At higher doses of sodium bisulfite, reduction of growth was noticed in this algal species. Among oxygen scavengers, sodium bisulfite is a common reducing agent and it readily reacts with dissolved oxygen [2]. Sodium bisulfite reacts with O2 and release sodium sulfate, SO2, and water molecules. Algal cultures treated with high dose exhibited low levels of total chlorophyll accumulation in this species [Figure 3]. Similarly, moderate concentration of NaHSO3 improved the growth in blue-green alga Anabaena, but high concentration inhibits the cell growth and photosynthesis and ultimately leads to death [2]. Probably, high concentration of scavengers may interact with other components in the TAP medium, and also, there is a chance of interruption of light penetration into the algal cells. Ma et al. [6] also proved that the addition of sodium bisulfite improves the growth and hydrogen production in C. reinhardtii at optimal dose.
3.3. Effect of Na2S2O4 onGrowth and Chlorophyll Accumulation in A. quadricellulare
In the current work, sodium dithionite was also used in various concentrations such as 0.0 mM, 0.16 mM, 0.32 mM, 0.40 mM, and 0.80 mM in the TAP medium to check the growth and biomass accumulation in A. quadricellulare [Figure 4]. Comfortable growth and high total chlorophyll content (44.46 mg/L) was noticed in this alga in TAP medium with 0.40 mM of sodium dithionite. In contrast, less growth was observed in A. quadricellulare at 0.80 mM. Based on the observation, it is confirmed that A. quadricellulare exhibited better resistance at optimal dose of sodium dithionite. Long back, Pow and Krasna [5] reported that the addition of sodium dithionite initiate low amount of photobiological hydrogen in green algae Scenedesmus obliquus and C. vulgaris. Kojima and Lin [27] also reported that the addition of sodium dithionite increases the hydrogen evolution (alternate pathway) in Chlorella pyrenoidosa which indicates its role in enhancement of biomass is limited up to certain dose. In addition, sodium dithionite was also used to estimate the H2ase activity in algal cultures [28].
 | Figure 2: Effect of Na2SO3 on growth (a) and chlorophyll accumulation and (b) in Asterarcys quadricellulare (Chl a-chlorophyll a, Chl b-chlorophyll b, and T Chl-Total chlorophylls). [Click here to view] |
 | Figure 3: Effect of NaHSO3 on growth (a) and chlorophyll accumulation and (b) in Asterarcys quadricellulare (Chl a-chlorophyll a, Chl b-chlorophyll b, and T Chl-Total chlorophylls). [Click here to view] |
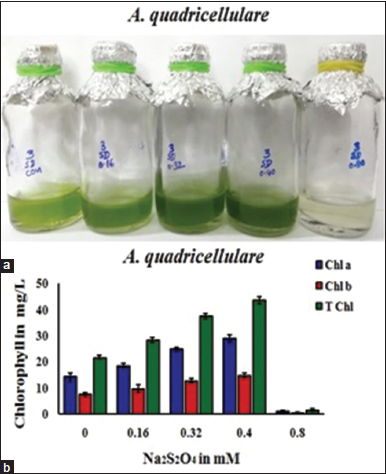 | Figure 4: Effect of Na2S2O4 on growth (a) and chlorophyll accumulation and (b) in Asterarcys quadricellulare (Chl a-chlorophyll a, Chl b-chlorophyll b, and T Chl-Total chlorophylls). [Click here to view] |
3.4. Effect of Na2S2O5 on Growth and Chlorophyll Accumulation in A. quadricellulare
Different concentrations of sodium metabisulfite, that is, 0.0 mM, 0.04 mM, 0.08 mM, 0.16 mM, 0.20 mM, and 0.40 mM were used in TAP medium to check the growth pattern and chlorophyll accumulation in this alga [Figure 5]. Augmented growth and total chlorophyll content (41.01 mg/L) in A. quadricellulare was observed in TAP with 0.08 mM sodium metabisulfite. At high concentration (0.40 mM), inhibition of algal growth was noticed. Till date, there was no report on oxygen scavenging capacity of sodium metabisulfite with algal cultures, and recently, our laboratory used it while working with C. minutum [3]. However, Lee et al. [29] developed the sulfite-based oxygen scavengers for the preservation of plant-based food materials specifically using sodium metabisulfite. Overall current investigation standardized the optimal concentration of oxygen scavengers in this alga, which will be helpful for other algal species specifically for anaerobic experiments.
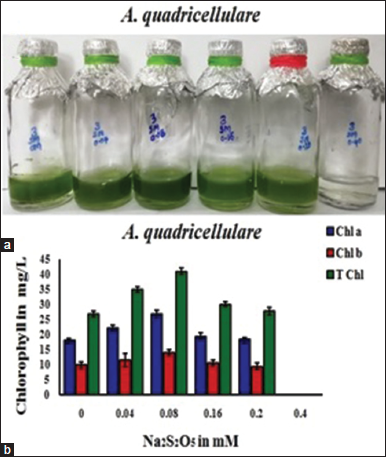 | Figure 5: Effect of Na2S2O5 on growth (a) and chlorophyll accumulation and (b) in Asterarcys quadricellulare (Chl a-chlorophyll a, Chl b-chlorophyll b, and T Chl-Total chlorophylls). [Click here to view] |
4. CONCLUSIONS
Oxygen scavengers initiate the anaerobic conditions by removing oxygen in any culture. Hydrogen production in algal culture is the main aim of the current investigation, but it occurs through activation of hydrogenase which, in turn, is oxygen-sensitive. Enhanced growth and total chlorophyll content (63.23 mg/L) was noticed in A. quadricellulare cultures grown in TAP with 3.2 mM of Na2SO3 medium. Similarly, augmented growth and total chlorophyll content (49.38 mg/L) was observed in A. quadricellulare cultures grown in TAP with 0.4 mM NaHSO3 medium. TAP with 0.4 mM of Na2S2O4 medium improved the growth and total chlorophyll content (44.46 mg/L) in this alga. Augmented total chlorophyll content (41.01 mg/L) in A. quadricellulare was observed in TAP with 0.08 mM Na2S2O5 medium. Higher doses of scavengers reduced or inhibited the growth in this alga. Present standardization will be helpful for anaerobic experiments in algal cultures.
5. ACKNOWLEDGMENTS
The authors are thankful to Prof. N. Mathivanan, Madras University, Chennai, India for providing algal cultures.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. FUNDING
There is no funding to report for this work.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
Not applicable for this work.
10. DATA AVAILABILITY
All the data are done in Yogi Vemana University, Kadapa, Andhra Pradesh.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Paramesh K, Lakshmana Reddy N, Shankar MV, Chandrasekhar T. Enhancement of biological hydrogen production using green alga Chlorococcum minutum. Int J Hydrogen Energy 2018;43:3957-66. CrossRef
2. Wang L, Chen M, Wei L, Gao F, Zhongxian LV, Wang Q, et al. Treatment with moderate concentrations of NaHSO3 enhances photobiological H2 production in the cyanobacterium Anabaena sp. strain PCC 7120. Int J Hydrogen Energy 2010;35:12777-83. CrossRef
3. Paramesh K, Chandrasekhar T. Improvement of photobiological hydrogen production in Chlorococcum minutum using various oxygen scavengers. Int J Hydrogen Energy 2020;13:7641-6. CrossRef
4. Nagarajan D, Lee DJ, Kondo A, Chang JS. Recent insights into biohydrogen production by microalgae – From biophotolysis to dark fermentation. Bioresour Technol 2017;227:373-87. CrossRef
5. Pow T, Krasna AI. Photoproduction of hydrogen from water in hydrogenase-containing algae. Arch Biochem Biophys 1979;194:413-21. CrossRef
6. Ma W, Chen M, Wang L, Wei L, Wang Q. Treatment with NaHSO3 greatly enhances photobiological H2 production in the green alga Chlamydomonas reinhardtii. Bioresour Technol 2011;102:8635-8. CrossRef
7. Wei L, Yi J, Wang L, Gao F, Wang Q, Ma W. Light intensity is important for hydrogen production in NaHSO3 treated Chlamydomonas reinhardtii. Plant Cell Physiol 2017;58:451-7. CrossRef
8. Juneja A, Ceballos RM, Murthy GS. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013;6:4607-38. CrossRef
9. Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N. Biofuels from microalgae. Biotechnol Progr 2008;24:815-20. CrossRef
10. Lee K, Eisterhold ML, Rindi F, Palanisami S, Nam PK. Isolation and screening of microalgae from natural habitats in the Midwestern United States of America for biomass and biodiesel sources. J Nat Sci Biol Med 2014;5:333-9. CrossRef
11. Satpati GG, Pal R. Microalgae-biomass to biodiesel: A review. J Algal Biomass Util 2018;9:11-37.
12. Satpati GG, Pal R. Co-cultivation of Leptolyngbya tenuis (Cyanobacteria) and Chlorella ellipsoidea (Green alga) for biodiesel production, carbon sequestration and cadmium accumulation. Curr Microbiol 2021;78:1466-81. CrossRef
13. Raga Sudha N, Varaprasad D, Bramhachari PV, Sudhakar P, Chandrasekhar T. Effects of various factors on biomass, bioethanol and biohydrogen production in green alga Chlamydomonas reinhardtii. J Appl Biol Biotechnol 2021;9:152-6.
14. Norziah MH, Ching Y. Nutritional composition of edible seaweeds Gracilaria changgi. Food Chem 2002;68:69-76. CrossRef
15. Varaprasad D, Raghavendra P, Raga Sudha N, Himabindu Y, Kumari MK, Parveen SN, et al. Augmentation of ethanol production in green alga Chlorococcum minutum through graphene oxide-supported platinum-ruthenium (Pt-Ru/RGO) nanoparticles. Bioenergy Res 2021. Doi: 10.1007/s12155-021-10282-4. CrossRef
16. Markou G, Angelidaki I, Georgakakis D. Microalgal carbohydrates: An overview of the factors influencing carbohy-drates production and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol 2012;96:631-45. CrossRef
17. Khoo HE, Prasad KN, Kong KW, Jiang Y, Ismail A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011;16:1710-38. CrossRef
18. Welfle A, Gilbert P, Thornley P. Increasing biomass resource availability through supply chain analysis. Biomass Bioener 2014;70:249-66. CrossRef
19. Cha KH, Lee HJ, Koo SY, Song DG, Lee DU, Pan CH, et al. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J Agri Food Chem 2010;58:793-7. CrossRef
20. Costache MA, Campeanu G, Neata G. Studies concerning the extraction of chlorophyll and total carotenoids from vegetables. Romanian Biotechnol Lett 2012;17:7702-8.
21. Subramanian S, Barry AN, Pieris S, Sayre RT. Comparative energetics and kinetics of autotrophic lipid and starch metabolism in chlorophytic microalgae: Implications for biomass and biofuel production. Biotechnol Biofuels 2013;6:1-12. CrossRef
22. Narravula N, Thummala C, Duddela V, Nandhagopal M, Narayanasamy M. Asterarcys quadricellulare isolate a1 small subunit ribosomal RNA gene, partial sequence. 2019 GenBank: MN179327.1
23. Arnon D. Copper enzymes isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol 1949;24:1-15. CrossRef
24. Varaprasad D, Narasimham D, Paramesh K, Raga Sudha N, Himabindu Y, Keerthi Kumari M, et al. Improvement of production of ethanol using green alga Chlorococcum minutum. Environ Technol 2021;42:1383-91. CrossRef
25. Collaco CR, Hochman DJ, Goldblum RM, Brooks EG. Effect of sodium sulfite on mast cell degranulation and oxidant stress. Ann Allergy Asthma Immunol 2006;96:550-6. CrossRef
26. Wei A, Xin X, Wang Y, Zhang C, Cao D. Signal regulation involved in sulfur dioxide-induced guard cell apoptosis in Hemerocallis fulva. Ecotoxicol Environ Saf 2013;98:41-5. CrossRef
27. Kojima E, Lin B. Effect of partial shading on photoproduction of hydrogen by Chlorella. J Biosci Bioeng 2004;97:317-21. CrossRef
28. Hwang JH, Kim HC, Choi JA, Abou-Shnab R, Dempsey B, Regan J. Photoautotrophic hydrogen production by eukaryotic microalgae under aerobic conditions. Nat Commun 2014;5:1-6. CrossRef
29. Lee JS, Jeong S, Lee HG, Cho CH, Yoo SR. Development of a sulfite-based oxygen scavenger and its application in kimchi packaging to prevent oxygen-mediated deterioration of kimchi quality. J Food Sci 2018;83:3009-18. CrossRef