1. INTRODUCTION
Agriculture, the main economic sector of many developing countries, is a sector most suffering from the impact of the ever-changing climate [1,2]. During the previous periods, the principal driving force of agriculture was increasing productivity. However, during the climate change period, increment in crop productivity should also be consider sustainability [3]. For which, legumes are chosen as a possible solution to agriculture’s sustainability during the climate change times as legumes contribute nitrogen (N) through symbiosis [4]. As legumes provide oil, fiber, and protein-rich food, besides their N contribution to the soil considered as the most important food source with sustainable and inexpensive meat substituting capacity [5,6].
In sub-Saharan African (SSA) countries, cowpea is most important crop among legumes for farmers, whose agricultural systems are largely deficient in plant nutrient, particularly N. This is because it performs under varying environments and fixed large quantity of N through biological N fixation (BNF) [7]. Therefore, cowpea can bring agronomic, environmental, and economic advantages for the rural poor [8]. Moreover, it exceeded most of the legumes to enhance soil fertility due to high N-fixing capacity when inoculated with effective rhizobia [9].
Nutritionally, cowpea provides high protein and carbohydrates, and it is also a source of vitamins, minerals, and micronutrients [10]. Due to its high nutritional value, it supports the rural poor in SSA, where carbohydrate-rich crops serving as a base for their diet [7,11]. Thus, gastrointestinal disorders [12], cardiovascular diseases, hypercholesterolemia, and obesity are among the diseases healed when cowpea is included in the diets [13]. Diabetes and several types of cancer can also be protected by the food sourced from cowpea [14]. Cowpea is important food source to regulate body weight [15], healthy digestion, and blood circulation systems [12].
Its ability to grow in a stressed environment and capacity to fix high amount of N are the reason for the environmental advantage from the crop [16,17]. Raising the yield of cowpea, therefore, is possible using mineral and biologically fixed N. However, due to the pressing climate change effect, and with the recent awareness regarding the environment polluting potential of mineral fertilizers, a focus on the BNF sourced N is increasing [7]. Cowpea has the capacity to fix atmospheric N2 under stressed environments [18] and reported to contribute about 240 kg N per hectare annually [19]. However, its N2-fixing efficiency and yield affected by several factors including biotic, edaphic, and weather variables [20]. Ultraviolet (UV) radiation, carbon dioxide, temperature, light, and moisture are the major climatic factors affecting N2-fixation process [21]. On the other hand, a rhizobial establishment can be inhibited by factors such as crop species and genotypes, competitive native microorganisms, and poor inoculant adhesion and survival [22].
Due to drought and warm weather adaptive capacity of cowpea, the crop is under production in the lowland areas of Ethiopia and the seeds, pods, and leaves used for consumption [23]. The early maturity behavior coupled with its drought tolerance capacity the crop plays a paramount role for risk aversion in the drought affected lowlands of Ethiopia [24]. Even if, the country is reported as a center of diversity for cowpea, it is not often mentioned in most of the previous studies dealing with cowpea production and productivity. Although about 66.5% of Ethiopia’s arable land suites for cowpea production, its yield remained below the potential [25]. According to Beshir et al. [24], knowledge gap on the manifold uses of cowpea, and sub-optimal crop husbandry including reliance on local varieties and absent of bio-inoculant use, climate and soil fertility factors are attributing to the low yield of the crop. Hence, the current review aimed to assess the cowpea production system and its response to the yield limiting factors and bio-inoculants to raise awareness toward exploiting its potential toward achieving food security.
2. METHODOLOGY
This review used desired scientific materials which are searched and prioritized based on their relevance to the topic of interest. The final review manuscript thereby, prepared by reviewing and synthesizing those selected scholarly materials retrieved from Scopus, Web of Sciences, Google Scholars, ResearchGate, etc. A search for literatures was not limited by time and geographical locations. However, a focus was paid for recently published articles and scholarly materials addressing cowpea producing countries from developing worlds. The key phrases used as a search cord associated to legume N contribution to soil fertility, legume responses to inoculation, cowpea nutritional composition, and factors affecting N2 fixation.
3. RESULTS AND DISCUSSION
3.1. Cowpea Production and Utilization
Across the world, about 45 countries have reported to produce cowpea with a total land allocation of 14.5 million ha per year and estimated 6.5 million metric tons production with 450 kg ha-1 global average yield [26]. The Western African countries accounted for 83.4% of the global production in 2016 [27] as the crop adapts in warm weather and drought conditions in the tropics where other legumes cannot grow [28]. Cowpea is suitably cultivated under mixed system due to its shade tolerance and successfully produced in poorly fertile soil as it fixes high amount of N [29]. Therefore, cowpea’s services go from dietary diversification to food security. Due to this, about 4.3%, 1.5%, and 5.8% growth for area coverage, yield, and production reported for SSA in their respective orders [26]. However, the yield remained low even compared with other legumes. However, Ojiewo et al. [30] projected a yield growth from about 6.2 million MT in 2010 to nearly 8.4 million MT by 2020 is for SSA.
In Ethiopia, the crop is under production in the northwest, eastern, central rift valley, and southern region of the country [23]. A national average national yield of about 0.4 t ha-1, and a yield ranges from 2.2 to 3.2 t ha-1 from improved varieties under optimal agronomic practices have been reported for Ethiopia [24]. In Ethiopia, cowpea is estimate to cover 69,500 ha of land with annual production of 55,600 tons [24]. Recently, cowpea is considered as the most important lowland pulse in Ethiopia, due to the interest to benefit from its multiples of roles as it is used for food, feed, soil fertility restoration, and income sources [31,32]. Ethiopian farmers cultivate cowpea mostly on sandy and marginal soil conditions, considering its capacity to perform under water and nutrient limited environments [23], and with sole, intercropped, and mixed cultures [33].
Dry grain and leaf of cowpea used as a high-protein sources of food and animal fodder in many countries of Africa. Leaves and immature pods have been exploited as vegetables, and different forms of food prepared from the seeds of cowpea [34]. The leaves can be consumed in a boiled, blanched, dried, or fermented forms [35]. This will play a paramount importance for fighting hunger, especially in SSA during those months from planting to harvesting when the farmers experience food shortage. Moreover, cowpea plays a role toward sustainable soil fertility improvement, particularly in smallholder farming systems, as it fixes high amount of N through BNF [36]. Therefore, the crop significantly contributes to the sustainability, which thereby serve to mitigate climate change [24].
3.2. Cowpea Nutritional Composition
Nutritionally, legumes often are called “poor people’s meat” as the legumes are rich in quality protein, carbohydrates, oil, fiber, and sucrose [37]. In the recent times, nutritional value of legumes is attracting the interest of people across the globe due to the increasing demand for healthy food as legumes contribute to healthy diet and treat metabolic diseases [38]. Cowpea nutritionally complements low-protein cereal and tuber crop staples and serves millions of people in the developing countries as the crop provides a major source of dietary protein [39]. The seeds of cowpea also identified for its richness for minerals and vitamins [40]. Not only its grain but also the leaf serves as an important source of food having high proximate composition in it [35] [Table 1]. In Ethiopia, although the grain is mainly used for consumption, the leaf has also found to be consumed in some parts of the country [23]. The combined use of the grain, leaf, and the green pod is already been observed in major cowpea growing regions of Ethiopia [24] [Table 2].
3.3. Legume Cowpea-N Contribution to Soil Fertility
In Africa, the main factor limiting crop yield is N deficiency [41,42]. N2-fixing legumes including cowpea are found to improve soil fertility through symbiotic N contribution [43]. The input for N to agricultural production systems is sourced from atmospheric N2 fixation or mineral fertilizer application. The critical problem the farmers are facing is the declining capacity of the soil for N supply [44]. On the other hand, the population is increasing drastically across the globe, inquiring for the production of more food, thereby increased N uptake. To maintain productivity at least at the current level or to improve it in the future, either N derived from mineral fertilizers, or BNF must replace the N removed in agricultural produce. However, the production and environmental cost of mineral fertilizer sources coupled with the poor utilization of N from mineral fertilizer by crops (rarely exceeding 50%) increased the interest toward symbiotic sources of N [45]. In this regard, cowpea, as a legume, contributes a lot in soil nutrient cycling, because of its nature to form symbiosis with Rhizobium bacteria with a reported contribution of 70–350 kg N ha-1 through BNF [46,47]. This indicates the importance of legumes symbiosis to enhance crop yield with economic and ecological sustainability [3]. In Ethiopia, soil fertility restoration is the top priority reasons why cowpea is incorporated in the farming systems [25].
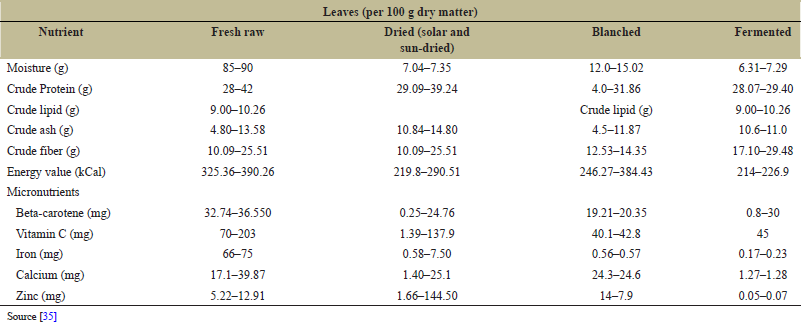 | Table 1: Cowpea, nutritional composition (mg/100 g dry weight) as a vegetable crop. [Click here to view] |
 | Table 2: Cowpea parts used for consumption in Ethiopia, presented as per regional administrative states (% respondents). [Click here to view] |
3.4. Effects of Rhizobial Inoculation on Growth and Photosynthesis
Crop production is vulnerable to climate change, indicating the urgent need for increased attention for improved agronomic practices including the use of bio-inoculants [48]. The low N contents of most tropical soil are an important factor in limiting crop growth and photosynthesis rates [49]. Thus, the supply of N to crops through symbiosis can increase leaf growth, photosynthesis, and net assimilation rates [50]. Being a legume, cowpea, meets its photosynthetic N requirements from symbiotic N2 fixation [51]. Inoculation with effective rhizobial strain found to significantly enhance development of cowpea in Ethiopian soils with an increase in the height of plants and dry mass of shoot in 12.4% and 31.3%, in their respective orders due to inoculation [52]. Significant improvement in cowpea leaf growth in terms of leaf area and leaf area index with inoculation has also been reported elsewhere [53,54]. Improvement in LAI due to inoculation leads to increased photosynthesis as the N nutrition enhances the synthesis of macromolecules responsible for CO2 interception and radiation capture, ribulose-1, 5-bisphosphate, and carboxylase-oxygenase (Rubisco), in their respective orders [55]. The improvement in photosynthesis rate in inoculated plants is achieved as symbiotic N supply promotes stomatal functioning, with reported improvement in leaf conductance of up to 24% with inoculation [56].
3.5. Effects of Inoculation on Agronomic Performance of Cowpea
Rhizobia are known to improve nodule development when legumes inoculated with effective strains [57]. In confirmation to this, a cowpea nodule number and dry weight increase of 67.3 and 77.1% due to inoculation with Bradyrhizobium reported in their respective orders [52]. However, nodule formation rate is firmly linked with the inherent soil fertility status. For instant, higher soil N will have an antagonistic effect on symbiosis, but without negative effect on yield [Figure 1a and b] [57]. Symbiotic N2 fixation by legumes is the important process in nature bringing in the atmospheric N to the soil with annually estimated amount of 35 million tons, an amount slightly exceeded by the amount of N supplied to the soil with the use of mineral fertilizers [58].
As legumes meet more than 70% of their N demand through symbiosis, the continuous N supply by BNF for plant growth and soil restoration has considerable contribution to environmental and economic sustainability [59]. Therefore, BNF is considered as a biological process playing a role for sustainability and serving as an alternative ways of environmental risk free production of food in the changing climate [60] [Table 3]. Among the legumes, cowpea has a huge role in maintaining soil health, as it found to contribute about 200 kg N per hectare [61]. Therefore, improvement in the assimilate production due to inoculation enhanced photosynthesis, which thereby leads to yield increment in cowpea [55]. A yield improvement of up to 2334 kg ha-1 [62] and 1223 kg ha-1 [63] was reported in cowpea when inoculated with effective rhizobial strains. Crops followed symbiotic legumes can also benefit as the fallen senescent leaves and belowground parts of the legumes enhance soil fertility [64]. For instance, sorghum yield advantage of 290% was reported when it succeeded cowpea crop [64].
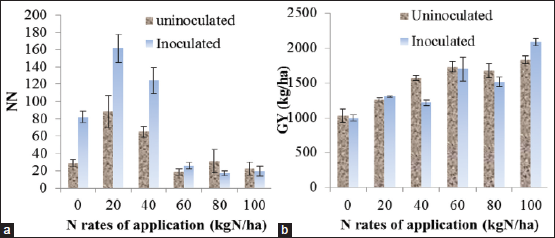 | Figure 1: Effect of nitrogen application rates (kg ha-1) on: (a) Nodule number, (b) grain yield (kg ha-1). Source: Saxena et al. [57] [Click here to view] |
Water is among the most important natural resources threatened by the clime change effect [65]. In this regard, improvements reported in legume crops water use efficiency (WUE) due to N2 fixation will have paramount importance when the water is becoming scarce during climate change periods [66]. A significant improvement in WUE of cowpea due to inoculation was reported elsewhere [67]. Improvement in WUE of inoculated cowpea may be associated with optimal nutrition, which thereby lead to healthier physiological functions and assimilation. Although several researches showed an increase in WUE with increased N nutrition, a contrasting finding, stating lower UWE in nodulated legumes was reported elsewhere [Figure 2] [68]. The difference in WUE between nodule forming legumes and the other plants is related with N fixing cost of plants [69].
3.6. Factors Affecting N2 Fixation
Sustainable agricultural systems development in the tropical N deficient soil largely linked to BNF by legumes. However, the legume root nodule formation and function in the tropics are constrained by different factor including climatic, soil, and management factors [73].
3.6.1. Soil and nutrition effects on N2 fixation
Excessive soil moisture, drought, soil acidity, phosphorus deficiency, and excess mineral N are the most important edaphic factors that limit BNF [73].
Waterlogging conditions inhibit root hair development and sites for nodule formation, and hinder O2 diffusion in plant roots. As logging decreases water activity below critical tolerance limits and indirectly alters plant growth, root architecture, and exudations, it influences the growth of rhizosphere microorganisms, like rhizobia [74]. Legumes in arid regions found to show poor nodulation performance and N2 fixation [74,75]. Several steps in the development of symbiosis, including the exchange of molecular signals between the legume and the microsymbiont, are affected by soil acidity, thereby affecting nodulation and N2 fixation [73]. However, naturally, there are rhizobia adapting to soil acidity [74], and the effect of soil acidity and aluminum toxicity on symbiosis can be reduced through liming treatment [73].
 | Figure 2: Relationships between the amounts of fixed nitrogen and water use efficiency. Source Brueck and Senbayram [68]. [Click here to view] |
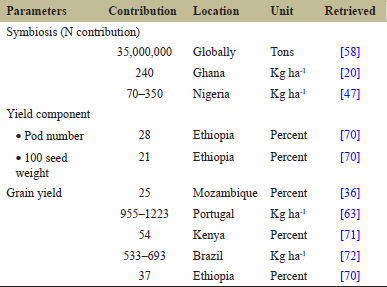 | Table 3: Effects of inoculation on cowpea symbiosis and agronomic performance. [Click here to view] |
Soil nutrient conditions have a significant effect on symbiosis, because survival and growth of rhizobia and host plant affected by soil nutritional condition [74]. Rhizobium infection process and N2 fixation in legumes inhibited by mineral N application [57] [Figures 1 and 3]. These two problems are because of impairment of the recognition mechanisms by nitrates, and due to diversion of photosynthates toward the assimilation of nitrates, respectively.
Although, large quantity mineral N fertilizer application inhibits N2 fixation, low doses of <30 kg N ha-1 mineral N fertilization can stimulate early growth of legumes thereby increase the overall N fixed [76]. For instance, a negative exponential relationship was observed between N2 fixation and mineral N fertilizer rate [Figure 3] [77]. In tropical Africa, phosphorus deficiency is common place and reduces nodule formation, symbiotic N fixation in legumes, and plant growth. This is because P enhances N2 fixation process due to its growth stimulating growth.
3.6.2. Climate change effects on N2 fixation
Climate change is already causing significant impacts on water resources and environmental. Accordingly, temperature, carbon dioxide concentration, and light (UV radiation) are among the important climatic factor affecting BNF [78]. The temperature has a marked influence on the survival and persistence of rhizobial strains in soils, thereby, adversely affect enzymatic process, N2 fixation [79]. Soil temperature also greatly influences competition for nodulation which might be due to a temperature-induced delay in nodulation or the restriction of nodules to the sub-surface region [80]. High root temperature influences rhizobial infection, N2 fixation ability, and legume growth [81]. The effect imposed on N2 fixation by higher temperature might be direct or indirect. This means that increased temperatures can directly decrease the nodule development [82], nodule activity [83], and hasten the senescence of nodules [84]. Even if nodules are formed, nodule might be ineffective and plants miss to accumulate N in shoots [81]. Temperature stress also affects the N2 fixation process through its effect on nodule forming rhizobia [85], with a maximum temperature of 32–47°C for their growth [73]. Therefore, it is reasonable to look for temperature stress tolerant strains of rhizobia to assure the success of symbiosis during the climate change time. In this regard, Bradyrhizobium reported to perform better than Rhizobium species under stressed conditions [86].
One of the changes happening during the climate change times is ozone depletion resulting in an increase in UV-B (280–315 nm) radiation at the Earth’s surface [87], with negative impacts on plants and animals [88]. The depletion of stratospheric O3 and consequent increase in the terrestrial UV-B radiation can cause deleterious effects on plants [89]. According to former researches on the UV-B effect, UV-B radiation has considerable photobiological consequences on the growth, development, and photosynthesis of plants [90]. The effect may be immediate on cowpea since the crop mainly produced in the tropical and subtropical regions, thinner ozone zone [89]. Raised UV-B radiation cause first-order effects including reductions in vegetative growth of plant and photosynthesis resulting with lower yield [91]. The sensitive nature of cowpea to UV-B radiation will aggravate the effect of the change on this crop calling for the identification and development of resistant varieties and agronomic technologies [92].
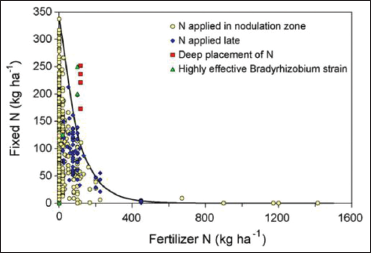 | Figure 3: Mineral nitrogen (n) supply versus fixed N, Source: Salvagiotti et al. [77]. [Click here to view] |
Numerous studies on CO2 enrichment have demonstrated an increase in productivity of most C3 crops with a doubling of [CO2] [93]. Legumes can overcome N supply limitation in future climate change times as elevated CO2 stimulates N2 fixation rate in their nodule symbionts [94]. Stimulation of N2 fixation by eCO2 can be driven by increasing nodule size and nodule numbers or stimulating nodule activity (amount of N2 fixed per unit of nodule mass) [93]. About 38% greater N2 fixation under elevated CO2, which attributed to the elevated CO2-induced stimulation of nodule number (33%), nodule biomass (39%), and nitrogenase activity (37%) were reported elsewhere [95]. Such stimulation of N2 fixation by elevated CO2 can result in a more proportion of total plant N coming from symbiotically fixed N, limiting the N uptake from the soil [96].
An increase in N uptake was reported in legumes with elevated CO2 concentration, which is linked to the increases in N fixed per legumes nodule in response to elevated CO2. The increase in N nutrition due to increased fixed N drives an increment in leaf chlorophyll content, nodulation, and N2 fixation, resulting in crop growth and biomass [97]. The increase in the growth performance improves the photosynthesis, leading to yield improvement under high CO2 concentration [Table 4] [98].
3.6.3. Biotic factors effect on N2 fixation
Quality of inoculants, ineffective nodules, leaf defoliation, and crop competition are among the biotic factors limiting the inoculants effectiveness [99]. The amount of N fixed by the legume – Rhizobium symbiosis influenced by the rhizobia used as a source of inoculation [99]. The major constraint in the N2 fixation process attributed to the absence of the required rhizobia species. This is because the competitive ability of an inoculated Rhizobium strain in comparison to indigenous strains, influences the proportion of the nodules formed on a particular host. Therefore, the introduced inoculum strain should outcompete the indigenous soil bacteria [100].
When the indigenous strains occupied the root nodules rather than the inoculum strains, the inoculation fails to improve legume productivity [101]. Although nodules were formed on the roots of legumes, some might not capable of fixing N2 [102]. For instance, in Australia, only 36% of the strains isolated from Acacia spp. found to significantly increase plant growth [103]. The important reasons for the formation of ineffective nodule can be (i) nodules are induced by incompatible rhizobia, (ii) nodules are induced by agrobacteria, or (iii) proper rhizobial strains are impaired in their symbiotic properties [104].
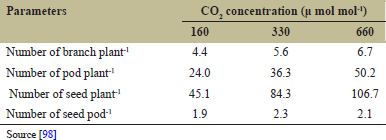 | Table 4: Yield attributing parameter under sub?ambient and superambient CO2. [Click here to view] |
Leaf defoliation of host plant, which decreases the photosynthetic ability of legumes, is also among the limiting biotic factors. Defoliation weakens symbiotic N fixation and also leads to decaying of nodules, since the assimilation process constrained by impaired photosynthesis. In perennial legumes, nodule decay sheds a high number of rhizobia in the root zone, and as new roots develop in subsequent growth cycles, nodule formation will be improved [105]. This will be particularly be a problem, for those legumes consumed as a leafy vegetable like cowpea, in Africa [105]. The nematodes can impose a competition on the rhizobia by affecting the root system of legumes [106].
3.7. Possible options to enhance N2-fixation in legumes
BNF in legumes represents N gain and serves toward mineral N fertilizer savings in cropping systems. Although legumes reported to fix about 250 kg N per hectare, the amount of N contributed through N2 fixation varies in time and space considerably. This is because the N fixation process is influenced by many factors as detailed in the former session, this, therefore, demands for application of management options to enhance biological fixation process. Inoculating with effectiveness proven strains and microbial screening for improved strains are the common approaches reported to enhance BNF. There are collections of effective rhizobia located at centers around in the world for most, if not all, legumes used in agriculture [107]. Identify the most effective and competitive one(s) through screening of these strains for a given agro-ecosystem may be important to optimize its uses. The identification of elite strains should be followed by the application of proper inoculation procedures to assure the service inoculation can offer.
N2 fixation in legumes can also be enhanced by selecting suitable host plants, cropping systems, and agronomic practices. Plant varieties with promiscuous nodulation to obviate the need for inoculation with rhizobia have already been developed by breeding programs. The potential benefit of screening these symbioses is underscored by the fact that only about 0.5% of existing leguminous species are presently used for agricultural purposes [108]. Agronomic management practices can also minimize some of the aforementioned factors limiting BNF. Mulching, for instance, can control weeds and fluctuations of soil moisture and temperature. Liming can eliminate soil acidity, and aluminum and manganese toxicities [73]. Some bacteria are able to promote plant growth through different mechanisms and they can do so endophytically, in symbiosis or as free-living cells. Plant growth-promoting bacteria may act directly by facilitating plant nutrients acquisition or influencing plant hormone levels, or indirectly by attenuating the inhibitory effects of pathogens [73].
4. CONCLUSION AND RECOMMENDATIONS
This review demonstrated the multiple roles of cowpea and its wide-ranging interaction with climate variables. Cowpeas have shown several agronomic, environmental, and economic advantages, contributing to further improve the diets and incomes of ruler farming communities. The crop performs well and most popular in the semiarid of the tropics where other food legumes do not perform potentially. It is a resilient crop and cultivated in extreme environments, which makes the cowpea a suitable choice during climate change times. BNF provides a sustainable supply of N for the growth of plants, add organic matter to the soil, and is it environmentally and economically sustainable sources of N. For such benefits, cowpea is an excellent choice due to its high N2-fixing potential. The major factors having a strong tie with BNF include temperature, elevated CO2, and biotic factors. Various studies on CO2 enrichment have demonstrated the N2 fixation stimulating effects of elevated CO2. In contrary, high temperature reported to cause reductions in the development and yield of crops by limiting the crop Bradyrhizobium symbiosis.
In Ethiopia, cowpea is primarily cultivated in lowland areas of the country and used as a source of food, feed, and soil fertility restoration. The leaves, pods, and seeds of cowpea are used as a source of food in Ethiopia. About 66.5% of Ethiopia’s arable land is reported to be suitable for the production of cowpea crop. However, the national average yield, annual production, and area coverage are limited to 400 kg ha-1, 55,600 tons, and 69,500 ha, respectively. Moreover, production and utilization of cowpea in Ethiopia are very limited compared to countries considered as major producers for cowpea. The availability of suitable arable lands and agro-ecological conditions coupled with the multiple role the crop can play calls for the expansion of cowpea production and utilization in the country. Therefore, introducing improved agronomic practices including varieties, and commercialization of inoculant technology is recommended to enhance cowpea productivity and optimize farmers benefit from the multifold purpose of cowpea.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
6. FUNDING
The publication is an output of a PhD scholarship at the Hawassa Universtiy, in the framework of the German-Ethiopian SDG Graduate School “Climate Change Effects on Food Security (CLIFOOD)” between the Food Security Center, University of Hohenheim (Germany) and the Hawassa University (Ethiopia), supported by the DAAD with funds from the Federal Ministry for Economic Cooperation and Development (BMZ).
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
Not applicable.
9. DATA AVAILABILITY
?Data included in article/supplementary material/referenced in article.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Kiprotich M, Mamati E, Bikketi E. Effect of climate change on cowpea production in Mwania Watershed: A case of Machakos County. I. J Educ Res 2015;3:1-12.
2. Mideksa K. Economic and distributional impacts of climate change: The case of Ethiopia. Glob Environ Change 2010;20:278-86.
3. Peoples M, Herridge, D, Ladha J. Biological nitrogen fixation: An efficient source of nitrogen for sustainable agricultural production. Plant Soil 1995;174:3-28.
4. Isgren E, Andersson E, Carton W. New perennial grains in African smallholder agriculture from a farming systems perspective. A review. Agron Sustain Dev 2020;40:6.
5. Jensen E, Peoples, M, Boddey RM, Gresshoff PM, Hauggaard-Nielsen H, Alves BJ, et al. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. Agron Sustain Dev 2011;32:329-64.
6. Arnon I. Field Beans (Phaseolus vulgaris L.) Crop Production in Dry Regions. 2nd ed. London: Leonard Hill; 1972. p. 245-247.
7. Chikowo R, Mapfumo P, Nyamugafata, Giller K. Mineral N dynamics, leaching and nitrous oxide losses under maize following two-year improved fallows on a sandy loam soil in Zimbabwe. Plant Soil, 2004;259: 315–30.
8. Singh B, Usha K. Nodulation and symbiotic nitrogen fixation of cowpea genotypes as affected by fertilizer nitrogen. J Plant Nutr 2003;26:463-73.
9. Ayanwuyi E, Akintonde JO. Effect of climate change on cowpea production in Kuje Area Council, Abuja, Nigeria. Int J Adv Res Manag Soc Sci 2012;1:273-83.
10. Ogbemudia FO, Denise EM, Ogie-Odia EA, Omonhinmin AC. Comparative germination studies of cowpea (Vigna unguiculata L.Walp.) and soy bean (Glycine max L. Merr.) on whole and water saturated fractions of hydrocarbon (hexane). Ann Biol Res 2010;1:34-40.
11. Jayathilake C, Visvanathan R, Deen A, Bangamuwage R, Jayawardana BC, Nammi S, et al. Cowpea: An overview on its nutritional facts and health benefits. J Sci Food Agric 2018;98:4793-806.
12. Indi T, Nicole NS, Wang AZ, Bollinger LB, Ngoma TN, Chimimba UK, et al. Common beans and cowpeas as complementary foods to reduce environmental enteric dysfunction and stunting in Malawian children: Study protocol for two randomized controlled trials. Trials 2015;16:520.
13. Frota KM, Mendonca S, Saldiva PH, Cruz RJ, Areas JA. Cholesterol lowering properties of whole cowpea seeds and its protein isolates in hamsters. J Food Sci 2008;73:235-40.
14. Barnes M, Uruakpa F, Udenigwe C. Influence of cowpea (Vigna unguiculata) peptides on insulin resistance. J Nutr Health Food Sci 2015;3:1-3.
15. Perera O, Liyanage R, Jayawardana BC, Vidanarachchi JK, Fernando P, Sivakanesan R. Modulating effects of cowpea incorporateddiets on serum lipids and serum antioxidant activity in Wistar rats. J Natl Sci Found Sri Lanka 2016;4:4-69.
16. Alexandre G, Piebiep G, Ana B, Domínguez-Perles R, Trindade H, Rosa EA, et al. Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable agric-food system: Nutritional advantages and constraints. J Sci Food Agric 2016;96:2941-51.
17. Sankie L, Addo-Bediako K, Ayodele V. Susceptibility of seven cowpea (Vigna unguiculata L. Walp) cultivars to cowpea beetle (Callosbruchusmaculatres). Agric Sci Res J 2012;2:65-9.
18. Antova, G, Stoilova T, Ivanova M. Proximate and lipid composition of cowpea cultivated in Bulgaria. J Food Compos Anal 2014;33:146-52.
19. Anteneh A, Abere M. Inherent soil fertility as affected by Rhizobium inoculation and inorganic N application on common bean. Ethiop J Agric Sci 2015;26:27-48.
20. Mohammadi K, Sohrabi Y, Heidari G, Khalesro S, Majidi M. Effective factors on biological nitrogen fixation. Afr J Agric Res 2012;7:1782-8.
21. Belnap J, Kaltenecke JH, Rosentreter R, Williams J, Leonard S, Eldridge D. Biological Soil Crusts: Ecology and Management. Technical Reference No. 1730-2. Denver, CO: USDI; 2001.
22. Niste M, Roxana V, Rodica P, Ioan R. Stress factors affecting symbiosis activity and nitrogen fixation by rhizobium cultured in vitro. ProEnvironment 2013;6:42-5.
23. Alemu M, Asfaw Z, Woldu Z, Fenta BA, Medvecky B. Cowpea (Vigna unguiculata (L.) Walp.) (Fabaceae) landrace diversity in northern Ethiopia. Int J Biodivers Conserv 2016;8:297-309.
24. Beshir B, Fenta BA, Teamir M, Tsegaye D, Yilma B, Ketema S. Cowpea Production, Marketing and Utilization in Ethiopia (Research Report 121, 2019). Ethiopian Institute of Agricultural Research; 2019.
25. Simion T. Adaptability performances of cowpea (Vigna unguiculata (L.) Walp) genotypes in Ethiopia. Food Sci Qual Manag 2018;72:43-7.
26. Abate T, Alene AD, Bergvinson D, Silim S, Orr A, Asfaw S. Tropical Legumes in Africa and South Asia: Knowledge and Opportunities (TL II Research Report No. 1, 2011). ICRISAT-Nairobi. Available from: https://hdl.handle. [Last accessed on 2021 Apr 25].
27. Food and Agriculture Organization. The State of Food Security in the World. Rome: Food and Agriculture Organization; 2010.
28. Ndiaye M, Spencer M, Gueye M. Genetic variability in dinitrogen fixation between cowpea [Vigna unguiculata (L.) Walp] cultivars determined using the nitrogen-15 isotope dilution technique. Biol Fertil Soils 2000;32:318-20.
29. Agbogidi OM. Screening six cultivars of cowpea (Vignia unguiculata (L.) Walp for adaptation to soil contaminated with spent engine oil. Acadamic Arena 2010;2:65-75.
30. Ojiewo CO, Rubyogo JC, Wesonga JM, Bishaw Z, Gelalcha SW, Abang MM. Mainstreaming Efficient Legume Seed Systems in Eastern Africa: Challenges, Opportunities and Contributions towards Improved Livelihoods. Rome: Food and Agriculture Organization of the United Nations; 2018. p. 72.
31. Ashinie SK, Tesfaye B, Wakeyo GK, Fenta BA. Genetic diversity for immature pod traits in Ethiopian cowpea [Vigna unguiculata (L.) Walp.] landrace collections. Afr J Biotechnol 2020;19:171-82.
32. Etana A, Tadesse E, Mengistu A, Hassen A. Advanced evaluation of cowpea (Vigna unguiculata) accessions for fodder production in the central rift valley of Ethiopia. J Agric Extens Rural Dev 2013;5:55-61.
33. Molla MA, Asfaw Z, Zerihun W, Fenta BA, Medvecky B. Cowpea (Vigna unguiculata (L.) Walp.) (Fabaceae) landrace diversity in Northern Ethiopia. Int J Biodivers Conserv 2016;8:297-309.
34. Ngalamu T, Odra J, Tongun N. Cowpea Production Handbook. Mumbai, India: IFS/AGRA, Afristar Publishing House; 2015.
35. Joshua O, George A, Okoth M, Mwang’ombe AW. A review of the contribution of cowpea leaves to food and nutrition security in East Africa. Food Sci Nutr 2020;8:36-47.
36. Kyei-Boahen S, Savala C, Chikoye D, Abaidoo R. Growth and yield responses of cowpea to inoculation and phosphorus fertilization in different environments. Front Plant Sci 2017;8:1-13.
37. Granito M, Paolini M, Perez S. Polyphenolsand antioxidant capacity of Phaseolus vulgaris storedunder extreme conditions and processed. LWT Food Sci Technol 2008;41:994-9.
38. Ade-Omowaye, Tucker, B, Smetanska I. Nutritional potential of nine underexploited legumes in Southwest Nigeria. Int Food Res J 2015;22:98-806.
39. Timko M, Singh B. Cowpea, a Multifunctional Legume, Genomics of Tropical Crop Plants. New York: Springer; 2008. p. 227-58.
40. Hall A, Cisseet N, Thiaw S, Elawad H, Ehlers J. Development of cowpea cultivars and germplasm by the Bean/Cowpea CRSP. Field Crops Res 2003;82:103-34.
41. Sumi A, Sugata S, Yahiro I, Odawara M. Effect of fertilizer and fixed nitrogen on the water use efficiency of Genge (Astragalus sinicus L.). Plant Prod Sci 2015;18:104-8.
42. Bationo A, Mokwunye A. Alleviating soil fertility constraints to increased crop production in West Africa: The experience of the Sahel. In: Mokwunye A, editor. Alleviating Soil Fertility Constraints to Increased Crop Production in West Africa. The Netherlands: Kluwer Academic Publishers; 1991. p. 195-215.
43. Bagayoko M, Buerkert A, Lung G, Bationo A, Römheld V. Cereal/legume rotation effects on cereal growth in Sudano-Sahelian West Africa: soil mineral nitrogen, mycorrhizae and nematodes. Plant Soil 2000;218:103-16.
44. Herridge D, Marcellos H, Felton W, Turner GL, Peoples MB. Chickpea increases soil-N fertility in cereal systems through nitrate sparing and N2 fixation. Soil Biol Biochem 1995;27:545-551.
45. Bohlool B, Ladha J, Garrity D, George T. Biological nitrogen fixation for sustainable agriculture: A perspective. Plant Soil 1992;141:1-11.
46. Zahran HH. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 1999;63:968-89.
47. Quin F. Advances in Cowpea Research. International Institute of Tropical Agriculture (IITA) and Japan International Research Center for Agricultural Sciences (JIRCAS); 1997. p. 9-14.
48. Olivares A, Carrillo-González R, González-Chávez MD, Hernández RM. Potential of castor bean (Ricinus communis L.) for phytoremediation of mine tailings and oil production. J Environ Manag 2013;114:316-23.
49. Belane AK, Dakora FD. Symbiotic N2 fixation in 30 field-grown cowpea (Vigna unguiculata L. Walp.) genotypes in the Upper West Region of Ghana measured using 15N natural abundance. Biol Fertil Soils 2010;46:191-8.
50. Ahmad S, Ahmad R, Ashraf MY, Ejaz A, Waraich A. Sunflower (Helianthus annuus L.) response to drought stress at germination and seedling growth stages. Pak J Bot 2009;41:647-54.
51. Hikosaka K, Terashima I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 1995;18:605-18.
52. Yoseph T, Baraso B, Ayalew T. Influence of Bradyrhizobia inoculation on growth, nodulation and yield performance of cowpea varieties. Afr J Agric Res 2017;12:1906-13.
53. Kaweski A. Genetically marked Rhizobuim identifiable as inoculum strains nodules plants grown in field populates with Rhizobuim japonicum. Environ Microbiol 1994;37:862-6.
54. Pereira S, Mucha A, Gonçalves B, Bacelar EA, Latr A, Ferreira HF, et al. Improvement of some growth and yield parameters of faba bean (Vicia faba) by inoculation with Rhizobium laguerreae and arbuscular mycorrhizal fungi. Crop Past Sci 2019;70:595-605.
55. Belane A, Dakora F. Assessing the relationship between photosynthetic C accumulation and symbiotic N nutrition in leaves of field-grown nodulated cowpea (Vigna unguiculata L. Walp.) genotypes. Photosynthetica 2015;53:562-71.
56. Augé RM, Toler HD, Saxton AM. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015;25:13-24.
57. Saxena A, Rathi S, Tilak K. Selection and evaluation of nitrate-tolerant strains of Rhizobium leguminosarum biovar viceae specific to the lentil. Biol Fertil Soils 1996;22:126-30.
58. Azam F, Farooq S. An appraisal of methods for measuring symbiotic nitrogen fixation in legumes. Pak J Biol Sci 2003;6:1631-40.
59. Yakubu H, Kwari J, Ngala A. N2 fixation by grain legume varieties as affected by rhizobia inoculation in the sandy loam soil of Sudano-Sahelian Zone of North Eastern Nigeria. Nig J Basic Appl Sci 2010;18:229-36.
60. Kessel C, Hartley C. Agricultural management of grain legumes: Has it led to an increase in nitrogen fixation? Field Crops Res 2000;65:165-81.
61. Dakora FD, Aboyinga RA, Mahama Y, Apaseku J. Assessment of N2 fixation in groundnut (Arachis hypogaea L.) and cowpea (Vigna unguiculata L. Walp) and their relative N contribution to a succeeding maize crop in Northern Ghana. Mircen J 1987;3:389-99.
62. Zilli J, Marson L, Marson B, Rumjanek NG. Contribution of rhizobia strains to cowpea development and grain yield in Roraima Brazil. Acta Amazonica 2009;39:749-57.
63. da Costa E, Nóbrega R, Martins A, Amaral FH, Moreira FM. Yield and nodulation of (Vigna unguiculata (L.) inoculated with rhizobia strains in Bom Jesus, PI. Rev Ciênc Agron 2011;42:1-7.
64. Bado B, Bationo A, Cescas M. Assessment of cowpea and groundnut contributions to soilfertility and succeeding sorghum yields in the Guinean savannah zone of Burkina Faso (West Africa). Biol Fertil Soils 2005;43:171-6.
65. Chatterjee S, Banerjee S, Bose M. Climate Change Impact on Crop Water Requirement in Ganga River Bais, West Bengal, India. Vol. 46. 3rd International Conference on Biology, Environment and Chemistry IPCBEE; 2012.
66. Xu L, Hsiao T. Predicted versus measured photosynthetic water-use efficiency of crop stands under dynamically changing field environments. J Exp Bot 2004;55:2395-411.
67. Moussa T, Chao W, Ximei Z. Leaf gas exchange, plant water relations and water use efficiency of Vigna unguiculata L. Walp. inoculated with Rhizobia under different soil water regimes. Water 2019;11:498.
68. Brueck H, Senbayram M. Low nitrogen supply decreases water-use efficiency of oriental tobacco. J Plant Nutr Soil Sci 2009;172:216-23.
69. Norimitsu H, Yuri U, Masayoshi T, Kumagai E, Araki T, Ueno O. Genetic variations in dry matter production, nitrogen uptake, and nitrogen use efficiency in the AA genome Oryza species grown under different nitrogen conditions. Plant Prod Sci 2013;16:107-16.
70. Ayalew T, Yoseph T, Petra H, Cadisch G. Yield response of field-grown cowpea (Vigna unguiculata (L.) Walp.) varieties to Bradyrhizobium inoculation. Agron J 2021;113:3258-68.
71. Onduru D, De Jager A, Muchena F, Gachini GN, Gachimbi L. Exploring potentials of rhizobium inoculation inenhancing soil fertility and agroeconomic performance of cowpeas in Sub-Saharan Africa. Am Eur J Sustain Agric 2008;2:185-97.
72. da Costa EM, Nóbrega RS, Martins L, Amaral FH, Moreira FM. Yield and nodulation of (Vigna unguiculata (L.) Walp.) inoculated with rhizobia strains in Bom Jesus, PI. Rev Ciênc Agron 2011;42:1-7.
73. Mariangela H, Milton V. Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Res 2000;65:151-64.
74. Abdullah M, Al-Falih K, Factors Affecting the Efficiency of Symbiotic Nitrogen Fixation by Rhizobium. Pakistan Journal of Biological Sciences 2002;5:277-1293.
75. Boscari A, Mandon K, Dupont L, Poggi MC, Le Rudulier D. BetS is a major glycine betaine/praline betaine transporter required for early osmotic adjustment in Sinorhizobium meliloti. J Bacteriol 2002;184:2654-63.
76. Ledgard SF, Steele KW. Biological nitrogen fixation in mixed legume/grass pastures. Plant Soil 1992;141:137-53.
77. Salvagiotti F, Cassman KG, Specht JE, Walters DT, Weiss A, Dobermann A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res 2008;108:1-13.
78. Magadza C. Climate change impacts and human settlements in Africa: Prospects for adaptation. Environ Monit Assess 2000;61:193-205.
79. Dietrich W, William E. Nitrogen Fixation in Agriculture, Forestry, Ecology, and the Environment. The Netherlands: Springer; 2005.
80. Eric WT, Michael JS. Genetics of competition for nodulation of legumes. Annu Rev Microbiol 1992;46:399-428.
81. Hungria M, Franco A, Sprent J. New sources of high-temperature tolerant rhizobia for Phaseolus vulgaris L. Plant Soil 1993;149:103-9.
82. Junior L, MA, Lima AST, Arruda JR, Smith DL. Effect of root temperature on nodule development of bean, lentil and pea. Soil Biol Biochem 2005;37:235-9.
83. Hernandez-Armenta R, Wien HC, Eaglesham AR. carbohydrate partitioning and nodul function in common bean after heat stress. Crop Sci 1989;29:1292-7.
84. Raësaënen LA, Lindstroëm K. The effect of heat stress on the symbiotic interaction between Sinorhizobium sp. and Acacia Senegal. FEMS Microbiol Ecol 1999;28:63-74.
85. Lima AS, Arruda JR, Smith DL. Effect of roottemperature on nodule development of bean, lentil and pea. Soil Biol Biochem 2005;37:235-9.
86. Eddie J, Dipen P, John G, Franck WL, Chang WS, Stacey G, et al. Transcriptional and physiological responses of Bradyrhizobium japonicum to desiccation-induced stress. J Bacteriol 2007;189:6751-62.
87. World Meteorological Organization. Scientific Assessment of Ozone Depletion: Global Ozone Research and Monitoring Project Report No. 52. Geneva, Switzerland: World Meteorological Organization; 2011. p. 516.
88. Correia C, Coutinho J, Bacelar E, Gonçalves BM, Björn LO, Pereira JM. Ultraviolet-B radiation and nitrogen affect nutrient concentrations and the amount of nutrients acquired by aboveground organs of maize. Sci World J 2012;2012:608954.
89. Timko MP, Singh B. Cowpea, a multifunctional legume. In: Moore PH, Ming R, editors. Genomics of Tropical Crop Plants. New York: Springer; 2008. p. 227-58.
90. McNamara A, Hill W. UV-B irradiance gradient affects photosynthesis and pigments but not food quality of perihyton. Fresh Water Biol 2000;43:649-62.
91. Zhao D, Reddy K, Kakani V, John R, Sullivan J. Growth and physiological responses of cotton to elevated carbon dioxide and ultraviolet-B radiation under controlled environment conditions. Plant Cell Environ 2003;26:771-82.
92. Krupa S. Elevated Ultraviolet (UV)-B Radiation and Agriculture. Georgetown, Texas, USA: Springer Verlag Heidelberg and Landes Bioscience; 1998. p. 105-31.
93. Rogers A, Ainsworth EA, Leakey A. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiol 2009;151:1009-16.
94. Rogers A, Gibon Y, Stitt M, Morgan PB, Bernacchi CJ, Ort DR. Increased C availability at elevated carbon dioxide concentration improves N assimilation in a legume. Plant Cell Environ 2006;29:1651-8.
95. Lam SK, Hao XY, Lin E, Han X, Norton R, Mosier AR, et al. Effect of elevated carbon dioxide on growth and nitrogen fixation of two soybean cultivars in Northern China. Biol Fertil Soils 2012;48:603-6.
96. Feng Z, Rutting T, Pleijel H, Wallin G, Reich PB, Kammann CI, et al. Constraints to nitrogen acquisition of terrestrial plants under elevated CO2. Glob Change Biol 2015;21:3152-68.
97. Dey S, Chakrabarti B, Prasanna R, Pratap D, Singh SD, Purakayastha TJ, et al. Elevated carbon dioxide level along with phosphorus application and cyanobacterial inoculation enhances nitrogen fixation and uptake in cowpea crop. Arch Agron Soil Sci 2017;63:1927-37.
98. Allen LH. Effects of Increasing Carbon Dioxide Levels and Climate Change on Plant Growth, Evapotranspiration, and Water Resources. Scottsdale, AZ, Washington DC: National Research Council, National Academy Press; 1990. p. 101-47.
99. Alemayehu D, Zerihun A, Solomon B. Limitations and strategies to enhance biological nitrogen fixation in sub-humid tropics of Western Ethiopia. J Agric Biotechnol Sustain Dev 2018;10:122-31.
100. Triplett EW. Construction of symbiotically effective strain of Rhizobium leguminosarum bv. trifolii with increased nodulation competitiveness. Appl Environ Microbiol 1990;56:574-6.
101. Adriana M. Overview and Case studies on Biological Nitrogen Fixation: Perspectives and Limitations, Paper Prepared for FAO Advances; 2016.
102. Odee DW, Haukka H, McInroy G, Sprent JI, Sutherland JM, Young JP. Genetic and symbiotic characterization of rhizobia isolated from tree and herbaceous legumes grown in soils from ecologically diverse sites in Kenya. Soil Biol Biochem 2002;34:801-11.
103. Barnet YM, Catt PC. Distribution and characteristics of root-nodule bacteria isolated from Australian Acacia spp. Plant Soil 1991;135:109-20.
104. Lindström LA., Räsänen, K. Effect of biotic and abiotic constraints on the symbiosis between Rhizobia and the tropical leguminous trees Acacia and Prosopis. Indian J Exp Biol 2003;41:1142-59.
105. Sangeeta P, Shikha G, Naleeni R. Unravelling the potential of microbes isolated from rhizospheric soil of chickpea (Cicer arietinum) as plant growth promoter. 3 Biotech 2019;9:277.
106. Marta L, Ana M, Solange O. Legume growth-promoting rhizobia: An overview on the Mesorhizobium genus. Microbiol Res 2014;169:2-17.
107. Takishima Y, Shimura J, Ugawa Y, Sugawara H. Guide to World Data Center on Microorganisms with a List of Culture Collections in the World. Saitama, Japan: WFCC World Data Center on Microorganisms; 1989.
108. Glick BR. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012;2012:963401.