1. INTRODUCTION
Salinity is one of the most devastating abiotic stresses that affect most stages of the plant life-cycle, such as, seed germination, seedling establishment, development, vegetative and reproductive growth stages [1]. Over the years, studies have shown that high salt content in soils significantly retarded plant growth by impairing most physiological and metabolic processes. These include nutritional disorders, water stress, ion toxicity, oxidative stress and reduced cell expansion and division [2-4]. At present, salinity is one of the most severe abiotic stresses affecting most horticultural and agronomic crops [5]. Germination of seeds can also be affected by salinity either by creating osmotic potential that inhibits water uptake, or by toxic effects of ions on the viability of embryo which, ultimately, results in cessation of sprout growth [6]. These osmotic and ionic stresses result in reactive oxygen species (ROS) production in seeds chloroplasts, mitochondria, and apoplastic space [7]. Oxidative stress during salinity results in peroxidation of the membrane, ion leakage, and damage to nucleic acids, cell membranes and the seed’s cellular structure, which, in turn, reduces seed quality and total yield of the a?ected crop [8].
Seed germination and seedling are critical stages during a plant’s life-cycle for healthy crop production. Seeds are very delicate during germination and can be easily damaged by stress [9]. Salinity stress causes membrane damage in seedling growth, reduces CO2 intake, and decreases hydrolytic enzyme activity, increasing lipid peroxidation level [8].
Application of 24-epibrassinolide (BR), a plant growth regulator, regulates physiological characteristics and could support seed germination under saline stress conditions. Previous studies have shown the anti-stress function of BR on seed germination and vigor [10,11]. It was similarly reported that BR significantly increased tomato seedling growth under low temperatures and weak light stress [12] and cucumber seedling growth under Ca (NO3)2 stress [13]. The efficacy of BR in promoting seed germination under stress conditions was also observed in the case of some commercially important forest tree species [14]. In all, BR increases antioxidant enzyme activity by increasing the expression of antioxidant genes, and adjusting nutrient accumulation to promote seed germination and seedling growth under saline stress [15].
Globally, Soybean (Glycine max L.) is one of the largest sources of vegetable seed oil and protein resource for humans. Salt stress has inhibited soybean seed germination and ultimately decreased its yield [16]. Salt stress tolerance, especially at the germination stage, determines better plant establishment in saline soils. High and uniform germination plus emergence are the critical determinants of soybean yield, especially under stressed soil conditions [16,17]. Some studies have evaluated the mechanisms underlying the inhibition effect of salt on seed germination of various crops including soybean. Few studies have addressed the possibilities of using plant hormones or their synthetic analogues to boost seed germination under a wide range of salinity stress. Here, we evaluated the potentials of BR for promoting soybean seed germination and emergence under various salinity concentrations based on several indices.
2. MATERIALS AND METHODS
2.1. Plant Materials and Preparation
Soybean seeds (Tachiyutaka-8424h) used in the experiment were carefully selected and disinfected with 5% sodium hypochlorite by soaking for 10 min and later rinsing 3 times with distilled water. The seeds were obtained from Denki nojo, Yamagata-Japan. Deionized water (1 litre) was used to dissolve 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 g of salt (NaCl) to develop various levels of salinity (1.56, 3.13, 4.69, 6.25, 7.81, 9.38, 10.94, 12.50, 14.06 and 15.63 dSm−1, respectively). The BR used was produced by Cayman Chemicals, Ellsworth-USA. The treatments studied were factorial combinations of two BR rates (0 and 0.5 ml) and eleven levels of salinity (0.00 [control], 1.56, 3.13, 4.69, 6.25, 7.81, 9.38, 10.94, 12.50, 14.06, and 15.63 dSm−1 NaCl), giving a total of 22 treatment combinations, arranged into a completely randomized design and replicated 3 times. The experimental soil, classified as coarse-textured Entisols and characterized by weak surface aggregation was sterilized at 120°C for 30 min for 3 consecutive days, using an autoclave device (Hirayama Hiclave HVE 50, Saitama-Japan). Eighty grams (80 g) of the sterilized soil was filled in 10 cm diameter Petri dishes, and each Petri dish was moistened with 10 ml of the different concentrations of saline water [18]. The electrical conductivity of the soil extract (0.11 dSm−1) was obtained using an electrical conductivity meter (CM-20E, TOA, Tokyo TOA Electronics Ltd., Japan). Deionized water with no salt served as the control. Ten seeds were sown in each Petri dish. The BR was dissolved based on the manufacturer’s recommended rate of 5.0 ml–1.666 ml of ethanol and applied (0.5 ml) to each Petri dish according to the treatment combinations. The Petri dishes were arranged randomly in growth chamber 3 (SANYO, MLT-350HT, Japan) at a relative humidity of 60%, and 25°C in the dark. The germination test was performed in two repeats for validity and reliability of data. Germination records were taken daily for ten (10) days. Seeds were considered to have germinated on the emergence of a visible, healthy white radicle.
2.2. Determination of Germination Variables
Daily observations on radicle emergence commenced from the 2nd day after sowing for 10 days.
Germination variables determined were as follows:
2.2.1. Dormancy phase (days)
These were the number of days before the emergence of sprouts.
2.2.2. Final germination rate (FGR) (%)
Final GR (FGR) was calculated by adding the daily rates of germinated sprouts from beginning to the end of the germination test.
2.2.3. Germination average time (GAT- days)
The average germination time (GAT) was calculated according to Scott et al. [19]:
Where:
T1=Number of days from the beginning of the experiment
N1=Number of seeds germinated per day
S=Total number of seeds germinated
2.2.4. Velocity of germination (VG)
The VG was estimated according to Hartmann et al. [20]:
Where:
A1=Number of seeds germinated
T1=Number of days of germination.
2.2.5. Germination rate (GR- days)
This was recorded as a summation of newly sprouted seeds per total number of days of germination:
GDR= Σ(ni/di)
Where:
ni = Daily germinated seed
di = Number of day
2.2.6. Germination percentage (%) (GP)
Seed GP was estimated using the following formula:
2.2.7. Daily germination (DG- %)
This was counted daily based on the number of newly sprouted seeds at each observation from day of germination per total number of seeds sown:
2.3. Statistics
The data were subjected to analysis of variance using GenStat software 15.1 Edition to partition the effects of the two factors and their interactions. Treatment means were compared using Duncan’s New Multiple Range Test at a 5% probability level. Since a statistical comparison of data from the two repeats showed no signi?cant difference, the data were pooled for the analysis.
3. RESULTS
3.1. Dormancy Phase/FGR
Although salinity and BR had no significant effect on the dormancy phase (DP), BR application reduced the number of days to first sprout compared with no BR application. Conversely, higher salt concentration (10.94–15.63 dSm−1) increased the DP of the seeds [Figure 1]. It also reduced the FGR significantly relative to the control (S = 0.00 dSm−1). The highest FGR was obtained in Sa (1.56 dSm−1), while the lowest was obtained in Sj (15.63 dSm−1) salt concentration [Figure 2a]. However, the FGR was significantly (P ≤ 0.05) enhanced by BR application [Figure 2b]. The interaction between salinity and BR showed that FGR was statistically at par with BR application irrespective of the salt concentration [Figure 2c].
3.2. GAT
There was no consistent trend in GAT with increases in salinity [Figure 3a]. However, seeds treated with 9.38 dSm−1 solution significantly had the longest GAT, and seeds under higher concentrations (14.06 and 15.63 dSm−1) had much lower GAT than those in the control treatment (0.00 dSm−1). Seeds treated with 6.25 dSm−1 salinity had the shortest (P ≤ 0.05) GAT [Figure 3a]. Across salinity levels, especially at 6.25 dSm-1, BR application reduced (P ≤ 0.05) the GAT relative to no application [Figure 3b and c].
3.3. VG
There was no consistent trend in the VG with successive increases in salinity [Figure 4a]. However, salinity at 3.13–9.38 dSm−1 significantly promoted the VG which significantly reduced at 10.94–15.63 dSm−1. At all salinity levels, BR application significantly (P ≤ 0.05) enhanced the VG relative to no application [Figure 4b-c]. Seeds germinated at higher concentrations of salt (12.50 to 15.63 dSm−1) without BR had the lowest VG, while those treated with BR and germinated at 4.69 dSm−1 significantly had the highest (P ≤ 0.05) [Figure 4c].
3.4. GR
The GR decreased as salinity increased [Figure 5a]. Seeds under 1.56 and 3.13 dSm−1 salinity had statistically similar GR with the control (0.00 dSm−1), while those under 4.69–15.63 dSm−1 salinity had much lower (P ≤ 0.05) rates. Broadly, the GR was severely impeded at higher salinity concentrations (12.50–15.63 dSm−1), and seeds treated with BR showed an increased (P ≤ 0.05) rate [Figure 5b]. The interaction between salinity and BR significantly affected the GR. At each salinity level, BR treated seeds had a higher (P ≤ 0.05) GR than the untreated seeds [Figure 5c]. For the BR treated seeds, 1.56–6.25 dSm−1 and 9.38 dSm−1 [Figure 5c] showed similar GR with those under 0.00 dSm−1 salinity.
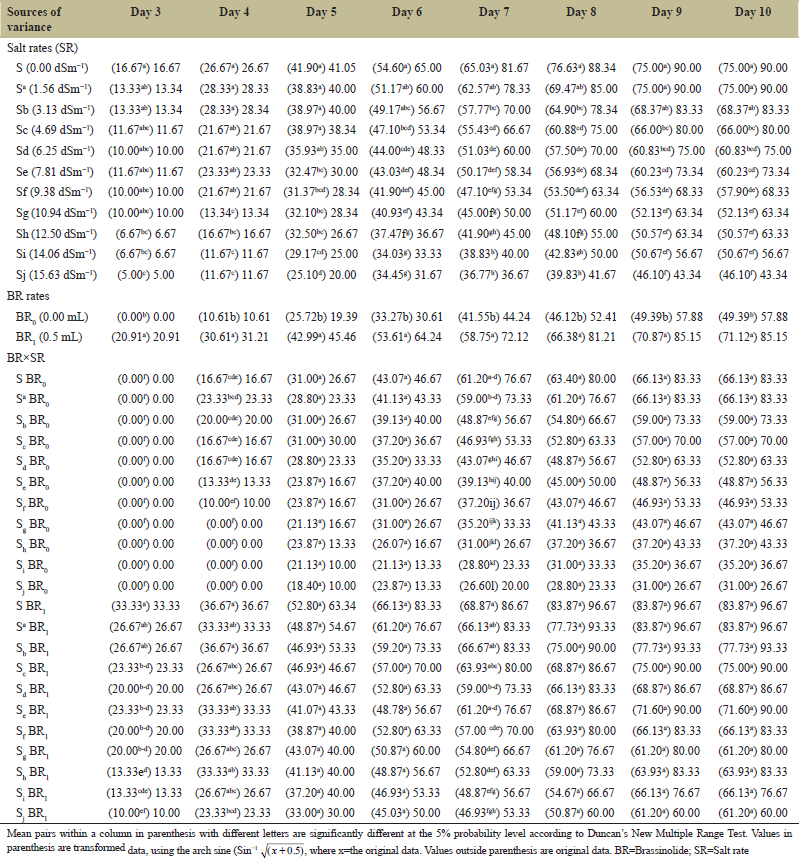
3.5. GP
The results of effect of salinity and BR on GP from the 3rd through the 10th day after sowing are presented in Table 1. Increases in salinity up to 10.94 dSm−1 (Day 3), 9.38 dSm−1 (Day 4), 6.25 dSm−1 (Day 5), 3.13 dSm−1 (Day 6), 1.56 dSm−1 (Days 7 and 8), and 3.13 dSm−1 (Days 9 and 10) had little effect on GP compared with the control (0.00 dSm−1). There was a significant (P ≤ 0.05) inhibition of germination at the higher salt concentration (12.50–15.63 dSm−1) compared with other concentrations on Days 5–10. For all the days of observation, BR treated seeds had a higher (P ≤ 0.05) GP than the untreated seeds, and there was no seed germinated on Day 3 for non-BR treated seeds. Higher salinity (14.06 to 15.63 dSm-1) reduced (P≤0.05) the GP, even with BR application compared with lower salinity (1.56–3.13 dSm−1) on Day 3. On Day 4 after sowing, BR application significantly enhanced GP at each salt level except at 1.56, 4.69, and 6.25 dSm−1 [Table 1]. Similarly, on Day 7 after sowing, the application significantly increased the GP at all salinity levels, except the control (0.00 dSm−1) and 1.56 dSm−1. The most salt-stressed seeds with no BR application had the lowest GP, while the highest GP was the non-stressed seeds treated with BR. In general, BR application enhanced seed GP at all salt concentrations [Table 1].
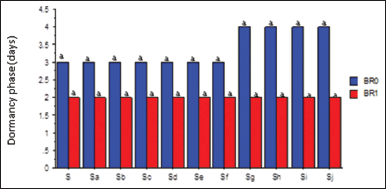 | Figure l: Salinity rates and BR interactions on dormancy phase of soybean seeds. BR0=No BR application (0 mL): BR1 = 0.5 mL: S = 0.00 dSm-1 (control); Sa = 1.56 dSm-1; Sb = 3.13 dSm-1; Sc = 4.69 dSm-1; Sd = 6.25 dSm-1; Se = 7.81 dSm-1; Sf = 938 dSm-1; Sg = 10.94 dSm-1; Sh = 12.50 dSm-1: Si = l4.06 dSm-1; Sj = 15.63 dSm-1. Mean pairs with different letter are significantly different at the 5% probability level according to Duncan’s’ New Multiple Range test. [Click here to view] |
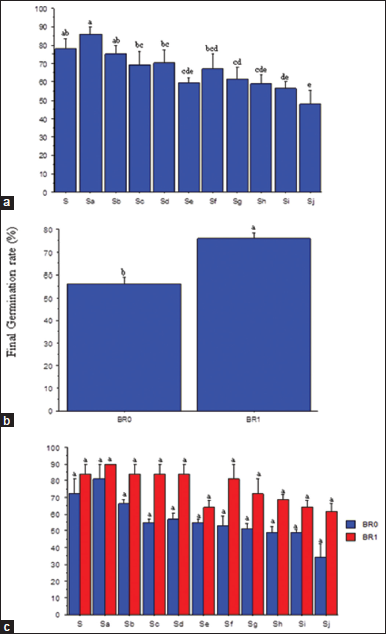 | Figure 2: Salinity rates (a) and brassinolide effects (b) and SR × BR interactions (c) on final germination rate of soybean seeds. BR0=No BR application (0 mL): BR1 = 0.5 mL: S = 0.00 dSm-1 (control); Sa = 1.56 dSm-1; Sb = 3.13 dSm-1; Sc = 4.69 dSm-1; Sd = 6.25 dSm-1; Se = 7.81 dSm-1; Sf = 938 dSm-1; Sg = 10.94 dSm-1; Sh = 12.50 dSm-1: Si = l4.06 dSm-1; Sj = 15.63 dSm-1. Mean pairs with different letter are significantly different at the 5% probability level according to Duncan’s’ New Multiple Range test. [Click here to view] |
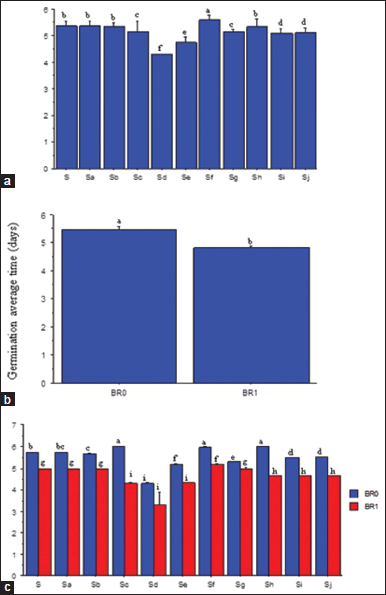 | Figure 3: Salinity rates (a), brassinolide effects (b) and SR × BR interactions (c) on germination average time of soybean seeds. SR: Salinity rate; BR=Brassinolide; BR0=No BR application (0 mL): BR1 = 0.5 mL: S = 0.00 dSm-1 (control); Sa = 1.56 dSm-1; Sb = 3.13 dSm-1; Sc = 4.69 dSm-1; Sd = 6.25 dSm-1; Se = 7.81 dSm-1; Sf = 9.38 dSm-1; Sg = 10.94 dSm-1; Sh = 12.50 dSm-1; Si = l4.06 dSm-1; Sj = 15.63 dSm-1. Mean pairs with different letter are significantly different at the 5% probability level according to Duncan’s’ New Multiple Range test. [Click here to view] |
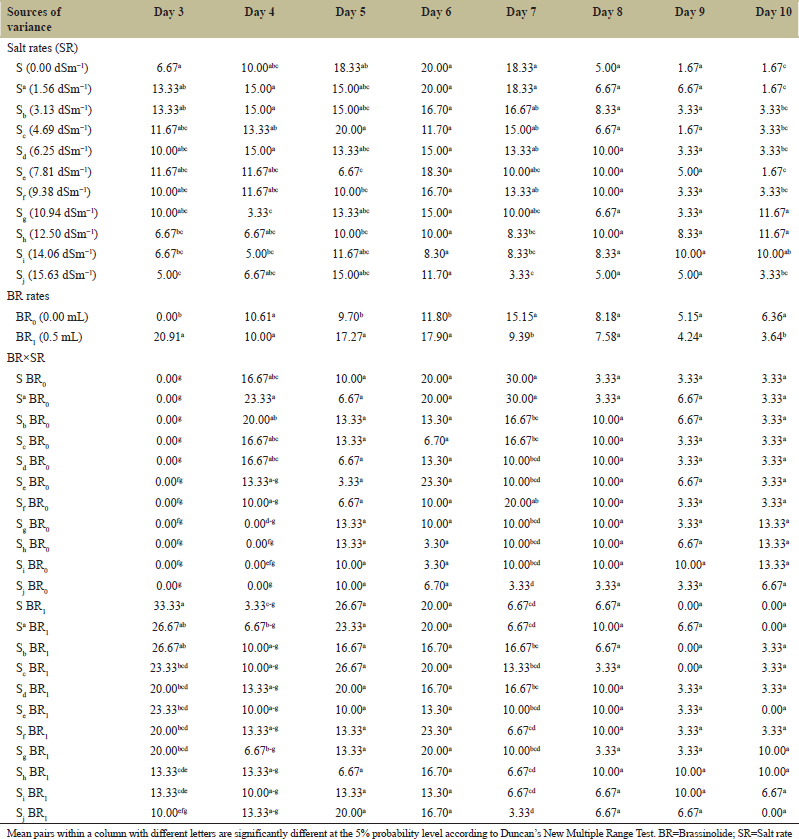
3.6. DG
The effects of BR and salinity on DG from Day 3 to Day 10 after sowing are shown in Table 2. On Days 3, 5, and 6 after sowing, BR application significantly enhanced DG compared with no application. However, on Days 7 and 10, DG was much (P ≤ 0.05) higher in seeds without BR application. Higher salt concentrations (12.50–15.63 dSm−1) significantly inhibited DG on Days 3 and 7. On Day 10, higher salinity (10.94–14.06 dSm−1) significantly increased DG relative to the control (0.00 dSm−1). On Day 3, seeds with no BR application, and those under 10.94–15.63 dSm−1 in Day 4 had no germination.
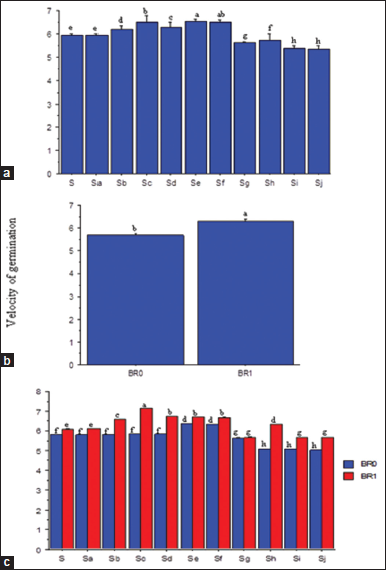 | Figure 4: Salinity rates (a), brassinolide effecta (b) and SR × BR interactions (c) on velocity germination of soybean seeds. SR: Salinity rate; BR=Brassinolide; BR0=No BR application (0 mL): BR1 = 0.5 mL: S = 0.00 dSm-1 (control); Sa = 1.56 dSm-1; Sb = 3.13 dSm-1; Sc = 4.69 dSm-1; Sd = 6.25 dSm-1; Se = 7.81 dSm-1; Sf = 938 dSm-1; Sg = 10.94 dSm-1; Sh = 12.50 dSm-1; Si = l4.06 dSm-1; Sj = 15.63 dSm-1. Mean pairs with different letter are significantly different at the 5% probability level according to Duncan’s’ New Multiple Range test. [Click here to view] |
4. DISCUSSIONS
Germination of seeds and good seedling emergence determine how well seedlings can survive in their natural environment. This constitutes the most critical stage in the life cycle of plants. Soybean seedling growth is often subjected to several abiotic stresses such as salinity, drought, extreme temperature, heavy metals, high radiation, and ?ooding [15-17]. These cause imbalances in cell homeostasis due to the generation of ROS, which induce membrane lipid peroxidation that leads to cell death [21]. A high concentration of sodium chloride is toxic to seeds and can inhibit germination due to low osmotic potential, ion toxicity and some biochemical and physiological dysfunctions [22]. A new group of phytohormones, BR, has been widely reported to confer some tolerance level and regulates seed germination and seedling growth under several abiotic stresses [15,23-27].
4.1. Dormancy Phase/FGR
Seed dormancy is a critical phase that predicts the time of seedling emergence based on environmental conditions at the time of seed production [28]. There was no significant effect of salinity and BR on the dormancy phase of soybean seeds, which contradicted the reports in Kan et al. [17] and Coll et al. [29]. However, successive increments in salt concentration significantly decreased the FGR of the soybean seeds. This could presumably be due to osmotic stress, being a major inhibitory factor that could hinder the uptake of water as a result of disruption of biochemical processes under saline condition [30]. Nizam [31] ascribed the delayed germination of oat seeds to low water absorption due to increased osmotic pressure. However, the application of BR significantly improved the FGR possibly because, as a principal phytohormone, it can control several important physiological activities, including seed germinations [32,33].
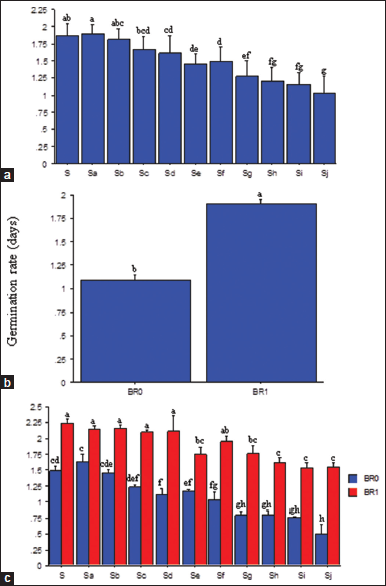 | Figure 5: Salinity rates (a), brassinolide effect a (b) and SR × BR interactions (c) on germination rate of soybean seeds. SR: Salinity rate; BR=Brassinolide; BR0=No BR application (0 mL): BR1 = 0.5 mL: S = 0.00 dSm-1 (control); Sa = 1.56 dSm-1; Sb = 3.13 dSm-1; Sc = 4.69 dSm-1; Sd = 6.25 dSm-1; Se = 7.81 dSm-1; Sf = 938 dSm-1; Sg = 10.94 dSm-1; Sh = 12.50 dSm-1; Si = l4.06 dSm-1; Sj = 15.63 dSm-1. Mean pairs with different letter are significantly different at the 5% probability level according to Duncan’s’ New Multiple Range test. [Click here to view] |
4.2. GAT
The duration of soybean seed germination was generally low, especially at the highest salinity level. This tendency may be attributed to damage to the seed membrane from the extreme generation of ROS [34]. Kumawat et al. [35] also reported that a decrease in the germination period could be attributed to increases in the concentration of salinity. The exogenous treatment of soybean seeds with BR boosted GAT, implying that BR could stimulate rapid and effective seed germination, even under the harmful effects of different abiotic stresses [36]. Brassinolide, as a phytohormone, can coordinate different signaling pathways during exposure to abiotic stresses and acts as a chemical messenger to regulate various cellular processes in seeds and seedling growth [37].
4.3. VG
As a determinant to crop growth and yield, VG expresses the GR in terms of the total number of seeds that germinated in a time interval. High salt concentration significantly reduced VG, partly due to oxidative stress that could have inhibited the speed of germination and prolonged germination. It could also be attributed to the disruptive effect of excessive salt on enzymatic structures and other macromolecules in the seeds, thereby causing damages to cell organelles and plasma membrane [38]. Despite its exposure to salt stress, brassinolide application enhanced early germination of soybean seeds. This could be attributed to its ability to regulate several genes related to cell division and differentiation [39], thereby activating various developmental processes, including early seed germination up to maturation [40].
4.4. GR
Seed germination is a distinctive physiological process that determines the success or failure of seedling growth. In this study, the minimum GR was observed at the higher salinity level, whereas an increase occurred with decreasing salinity concentrations. This trend confirmed a previous report [41] that limitations in GR at higher salt concentrations may be due to loss of turgor and reduction in energy needed for sprout growth. A recent finding [42] also affirmed that higher GR at a lower salinity level could facilitate a faster and higher sprouts. The use of BR enhanced GR. This observation supported a previous report [29] that BR can reduce the inhibitory and harmful effects of salinity on seed germination.
4.5. GP
During germination assessment (days 3–10), there was no remarkable effect of salinity on GP. However, a significant reduction was observed at higher salt concentrations (12.50–15.63 dSm−1) relative to lower concentrations at earlier assessment days. This drastic reduction in GP could be attributed to saline shock, resulting from the osmotic effect of excessive sodium chloride during the onset of the germination process [43]. The high salt concentration may have altered water absorption due to the lower osmotic potential of the germination media. Furthermore, seeds exposed to the highest salt concentration without BR, had the least GP probably due to adverse ion toxicity effect on enzymatic activities of nucleic acid metabolism, protein metabolism, and the reduction in utilization of reserved food [44] for seed germination. Application of BR significantly improved GP across the germination assessment days. Recently, it was reported [45] that BR could mitigate the adverse effect of several stressors, including salinity stress on seed germination.
4.6. DG
Observing the DG trend is an essential indicator for evaluating salt tolerance during seed germination [46]. High salt concentrations significantly inhibited DG, especially on days 3, 4 and 7. Inhibition in DG could mainly be due to salinity-induced ionic imbalance that may have caused toxicity from osmotic effects [42] and delayed enzymatic activity that could hinder the release and development of radicles. A decrease in DG during salinity stress was similarly reported in Chauhan et al. [47]. Supplementation of saline solution with exogenously applied BR considerably reduced the inhibitory effect of salinity on DG, especially on days 3, 5, and 6 compared with no BR. Enhancement in DG could possibly be associated with enhanced levels of nucleic acids and soluble proteins, as nucleic acid and protein syntheses are two early processes that BR could effectively activate during germination and seedling growth [48].
 | Table 1: Main and interaction effects of brassinolide (BR) and sodium chloride (NaCl) concentrations on germination percentage (GP) of soybean seeds on 3–10 days after sowing. [Click here to view] |
 | Table 2: Main and interaction effects of brassinolide (BR) and sodium chloride (NaCl) concentrations on daily germination (DG) of soybean seeds on 3 to 10 days after sowing. [Click here to view] |
5. CONCLUSION
The results provided some evidence of the importance of the pre-sowing treatment of soybean seeds with BR under various saline concentrations. Salinity and BR significantly affected the germination variables of soybean seeds. Seed treatment with BR improved the tolerance of saline stress and ensured harmonized germination by breaking dormancy and enhancing viability. No significant difference in the dormancy phase was observed among salinity levels and BR treatments. Interestingly, the overall results indicated that increasing salinity levels significantly inhibited the germination indices (FGR, GAT, VG, GR, GP, and DG) across the assessment periods. However, the application of BR did significantly enhanced the germination variables and confirmed that seed pre-treatment with BR is an effective technique to improve soybean seed germination for better crop establishment under saline microclimates. Thus, BR could be tested for its germination enhancing potentials in other crops that are susceptible to salinity stress. Farmers planting soybean can be encouraged to use BR to enhance seed germination and plant establishment in saline affected soils.
6. AUTHORS’ CONTRIBUTIONS
All authors have read and agreed to the published version of the manuscript. Concept and analysis/Interpretation, U.I., O.V. and E.E.A.; Drafting manuscript, O.V. and U.I.; Critical review of manuscript, E.E.A., U.I. and A.P.; Statistical analysis, U.I. and I.M.O.; Funding, A.P.; Admin, technical or material support, A.P., E.E.A., L.S., S.Y. and I.M.O.; Supervision, A.P., E.E.A. and U.I.; Final approval, E.E.A., A.P., U.I. and O.V.
7. ACKNOWLEDGMENTS
The authors acknowledge the management in-charge of the Joint Research Associate Program of the Arid Land Research Center, Tottori University, Tottori-Japan, who partly supported this research to be successfully conducted.
8. FUNDING
The Research was partly funded by the Arid Land Research Center (ALRC), Tottori University, Tottori, Japan.
9. CONFLICT OF INTERESTS
We declare no conflict of interests regarding the publication of this manuscript.
10. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
11. DATA AVAILABILITY
Data generated in this research are presented therein.
12. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Zhu JK. Abiotic stress signaling and responses in plants. Cell 2016;167:313-24. CrossRef
2. Munns R. Comparative physiology of salt and water stress. Plant Cell Environ 2002;25:239-50. CrossRef
3. Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, et al. The MKK2 pathway mediates cold and salt signaling in Arabidopsis. Mol Cell 2004;15:141-52. CrossRef
4. Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 2016;7:1787. CrossRef
5. Nasrin S, Mannan MA. Impact of salinity on seed germination and seedling growth of tomato. J Biosci Agric Res 2019;21:1737-48. CrossRef
6. Lianes A, Reinoso H, Luna V. Germination and early growth of Prosopis strombulifera seedlings in different saline solutions. World J Agric Sci 2005;1:120-8.
7. Khan MIR, Iqbal N, Masood A, Khan NA. Variation in salt tolerance of wheat cultivars: Role of glycine-betaine and ethylene. Pedosphere 2012;22:746-54. CrossRef
8. Hessini K, Ferchichi S, Youssef SB, Werner KH, Cruz C. How does salinity duration a?ect growth and productivity of cultivated barley? Agron J 2015;107:174-80. CrossRef
9. Lapik YR, Kaufman LS. The Arabidopsis cupin domain protein AtPirin1 interacts with the G protein α-sub unit GPA 1 and regulates seed germination and early seedling development. Plant Cell 2003;15:1578-90. CrossRef
10. Nee G, Xiang Y, Soppe WJ. The release of dormancy, a wake-up call for seeds to germinate. Curr Opin Plant Biol 2016;35:8-14. CrossRef
11. Shu k, Liu XD, Xie Q, He ZH. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol Plant 2016;9:34-45. CrossRef
12. Shu S, Tang Y, Yuan Y, Sun J, Zhang M, Guo S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined temperature and weak light stress. Plant Physiol Biochem 2016;107:344-53. CrossRef
13. Yuan L, Yuan Y, Du J, Sun J, Guo S. Effect of 24-epibrassinolide on nitrogen metabolism in cucumber seedling under Ca(NO3)2 stress. Plant Physiol Biochem 2012;61:29-35. CrossRef
14. Kuneš I, Balaš M, Linda R, Gallo J, Novakova O. Effects of brassinosteroid application on seed germination of Norway spruce, scots pine, Douglas fir and English oak. iForest 2017;10:121-7. CrossRef
15. Otie V, Udo I, Shao Y, Itam MO, Okamoto H, An P, et al. Salinity effects on morpho-physiological and yield traits of soybean (Glycine max L.) as mediated by foliar spray with brassinolide. Plants 2021;10:541. CrossRef
16. Zhang WJ, Niu Y, Bu SH, Li M, Feng JY, Zhang J, et al. Epistatic association mapping for alkaline and salinity tolerance traits in the soybean germination stage. PLoS One 2014;9:e84750. CrossRef
17. Kan G, Ning L, Li Y, Hu Z, Zhang W, He X, et al. Identification of novel loci for salt stress at the seed germination stage in soybean. Breed Sci 2016;66:530-41. CrossRef
18. Rao NK, Hanson J, Dulloo ME, Gohosh K, Nowell D, Larinde M. Handbooks for Genebanks. No 8: Manual of Seed Handling in Genebanks. Rome: Bio-Diversity International; 2006. Available from: http://www.bioversityinternational.org/index.php?id=19&user_bioversitypublications_pi1[showuid]=3020 [Last accessed 2021 Oct].
19. Scott SJ, Jones RA, Williams WA. Review of data analysis methods for seed germination. Crop Sci 1984;24:1192-8. CrossRef
20. Hartmann HT, Kester DE, Davies FT Jr., Genene RL. Plant Propagation. 6th ed. New Delhi: Prentice Hall of India Private Ltd.; 1997.
21. Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 2020;7:405-10. CrossRef
22. Lin J, Wang Y, Sun S, Mu C, Yan, X. E?ects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci Total Environ 2017;576:234-41. CrossRef
23. Ramakrishna B, Rao SS. 24-Epibrassinolide maintains elevated redox state of AsA and GSH in radish (Raphanus sativus) seedlings under zinc stress. Acta Physiol Plant 2013;35:1291-302. CrossRef
24. Fariduddin Q, Ahmed M, Mir BA, Yusuf M, Khan TA. 24-epibrassinolide mitigates the adverse effects of manganese induced toxicity through improved antioxidant system and photosynthetic attributes in Brassica juncea. Environ Sci Pollut Res Int 2015;22:11349-59. CrossRef
25. Otie V, Ping A, Eneji E. Interactive effect of brassinolide and lime on growth and yield of maize (Zea mays L.) on acid soils of South-East Nigeria. Commun Soil Sci Plant Anal 2018;49:2918-31. CrossRef
26. Otie V, Ping A, Ibrahim A, Eneji E. Plant growth regulator-brassinolide for mitigating field waterlogging stress on maize. Int J Plant Soil Sci 2019;30:1-14. CrossRef
27. Lv J, Zong XF, Ahmad AS, Wu X, Wu C, Li YP, et al. Alteration in morpho-physiological attributes of Leymus chinensis (Trin.) Tzvelev by exogenous application of 24-epibrassinolide under varying levels of drought stress. Chil J Agric Res 2020;80:61-71. CrossRef
28. Gioria M, Pyšek P. Early bird catches the worm: Germination as a critical step in plant invasion. Biol Invasions 2017;19:1055-80. CrossRef
29. Coll Y, Coll F, Amorós A, Pujol M. Brassinosteroids roles and applications: An update. Biologia 2016;70:726-32. CrossRef
30. Mwando E, Han Y, Angessa TT, Zhou G, Hill CB, Zhang XQ, et al. Genome-wide association study of salinity tolerance during germination in Barley (Hordeum vulgare L.). Front Plant Sci 2020;21:118. CrossRef
31. Nizam I. Effects of salinity stress on water uptake, germination and early seedling growth of perennial ryegrass. Afr J Biotechnol 2011;10:10418-24. CrossRef
32. Li QF, Wang JD, Xiong M, Wei K, Zhou P, Huang LC, et al. iTRAQ-based analysis of proteins co-regulated by brassinosteroids and gibberellins in rice embryos during seed germination. Int J Mol Sci 2018;19:3460. CrossRef
33. Zhao X, Dou L, Gong Z, Wang X, Mao T. BES1 hinders ABSCISIC ACID INSENSITIVE5 and promotes seed germination in Arabidopsis. New Phytol 2019;221:908-18. CrossRef
34. Ibrahim EA. Seed priming to alleviate salinity stress in germinating seeds. J Plant Physiol 2016;192:38-46. CrossRef
35. Kumawat S, Gothwal DK, Kumawat RK, Kumawat R, Sharma M. Effect of salt stress on seed germination and early seedling traits in fenugreek (Trigonella foenum-graecum L.) genotypes grown under different salinity levels. J Pharmacogn Phytochem 2017;6:776-81.
36. Hasanuzzaman M, Fotopoulos V. Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants. Singapore: Springer; 2019.
37. Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci 2015;20:219-29. CrossRef
38. El-Hendawy S, Elshafei A, Al-Suhaibani N, Alotabi M, Hassan W, Dewir YH, et al. Assessment of the salt tolerance of wheat genotypes during germination stage based on germination ability parameters and associated SSR markers. J Plant Interact 2019;14:151-63. CrossRef
39. Li J, Yang P, Kang J, Gan Y, Yu J, Calderón-Urrea A, et al. Transcriptome analysis of pepper revealed a role of 24-epibrassinolide in response to chilling. Front Plant Sci 2016;7:1-16. CrossRef
40. Oh E, Zhu JY, Wang ZY. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol 2012;14:802-9.
41. Marcum KB. Use of saline and non-portable water in the turfgrass industry: Constraints and developments. Agric Water Manage 2006;80:132-46. CrossRef
42. Panuccio MR, Jacobsen SE, Akhtar SS, Muscolo A. Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB Plants 2014;6:plu047. CrossRef
43. Morales D, Potlakayala S, Soliman M, Daramola J, Weeden H, Jones A, et al. Effect of biochemical and physiological response to salt stress in Camelina sativa. Commun Soil Sci Plant Anal 2017;48:716-29. CrossRef
44. Wu GQ, Jiao Q, Shui QZ. Effect of salinity on seed germination, seedling growth, and inorganic and organic solutes accumulation in sunflower (Helianthus annuus L.). Plant Soil Environ 2015;61:220-6. CrossRef
45. Ali B. Practical applications of brassinosteroids in horticulture: Some field perspectives. Sci Hortic 2017;225:15-21. CrossRef
46. Li W, Zhang H, Zeng Y, Xiang L, Lei Z, Huang Q, et al. A salt tolerance evaluation method for sunflower (Helianthus annuus L.) at the seed germination stage. Sci Rep 2020;10:10626. CrossRef
47. Chauhan A, Rajput N, Kumar D, Kumar A, Chaudhry AK. Effect of different salt concentration on seed germination and seedling growth of different varieties of oat (Avena sativa L.). Int J Informat Res Rev 2018;3:2627-32. CrossRef
48. Wu W, Zhang Q, Ervin EH, Yang Z, Zhang X. Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-epibrassinolide. Front Plant Sci 2017;8:1017. CrossRef