1. INTRODUCTION
Polyphenol oxidases (PPOs) are proteinase enzymes that catalyze the oxidation of phenolic compounds and found in all living organisms; in insect, they are responsible for the exoskeleton, in animals, tyrosinase has a great role in wound healing, in fungi, has a role in spore formation and pigmentation, and a role in lignin degradation [1]. PPOs were used in the food industry for the improvement of flavor in tea, cocoa, coffee production, and ascorbic acid determination [2]. In environmental technology, PPOs are used in remediation for the removal of phenolic and the toxic compounds from wastewater [3]. In a medical application, PPO is used as a tumor-suppressing [4] and treatment of Parkinson’s disease [3].
PPOs are subdivided into three subclasses, tyrosinases, catechol oxidase, and laccase. They catalyze the oxidation of diphenolic compounds in the presence of atmospheric oxygen to produce quinones [4]. In the final stage, polymerization of these quinones to produce various pigments (black, brown, red) is the main reason for fruit browning and ripening in the plants. PPOs are copper-containing enzymes found mainly in the chloroplasts and mitochondria [5]. It was reported that the PPOs vastly valuable biocatalysts for several molecular applications [6]. Enzymatic browning of vegetables and fruits will affect their quality [7] and is considered as a very serious problem during the processing and storage of foods. The discoloration of food browning may affect the quality and cause some changes in color, flavor, taste, and nutritional [8].
In animals and humans, polyphenolic compounds play a role in immune defense by darkening of tissue during lesion formation and in healing wounds, infected tissues, and prevent further spread of pathogens.
Plants are considered as rich sources of phenolic compounds, which can act as antioxidant, antimicrobial, anti-inflammatory, and antidiabetic agents. Plant phenolic comprises simple phenols, such as coumarins, tannins, phenolic acids, and flavonoids [9]. The mainly common phenolic compounds that existed in plant organs are flavonoids. PPO had been widely investigated in many plant types, such as potato and taro [10], eggplant, pears [11], grapes [12], tea leaves, and pomegranate [13]. Some polyphenolic compounds have chemical and biological activities, such as antioxidants, anti-inflammatory, hepatoprotective, and antigenotoxic [14].
In the present study, the relationship between time and browning was determined in the following fruits of : Paradise apple (Pyrus malus L)., cucumber (Cucumis sativus L.), squash (Cucurbita pepo L.) rind and seeds of the pomegranate (Punica granatum L.), and the leaf of cactus (Opuntia ficus-indica (L.) Mill). The influence of pH and temperature on PPO activity was also investigated. Finally, the activity and kinetic parameters of the PPO enzyme in the studied plants were investigated too.
2. MATERIALS AND METHODS
2.1. Materials
All plants are purchased from the local markets (Jordan). Sodium fluoride is purchased from CODEX LTD Carloerba, Milan, Italy. Catechol and L-tyrosine all are purchased from BDH Laboratory Supplies, Poole, England. Di-sodium hydrogen phosphate anhydrous was purchased from Fluka-Chemieka AGCH9470 Buchy. Sodium-dihydrogen phosphate was purchased from Panreac, Montplet & Esteban SA, Barcelona-Espana. Sodium acetate was purchased from Riedel-Dehaen, Sigma-Aldrich Laborchemilealien GmbH Seelze.
2.2. Crud Enzyme Preparation
Fifty grams of fresh fruits and leaves of the studied plants (Table 1) were homogenized with the addition of sodium fluoride solution (1:5 w/v) in an electric food processor for 3 minutes. Homogenates were filtered and centrifuged to 10 minutes. The pellet was removed and the supernatant was used as crude plant extract (enzyme solution) and was kept at 4°C [15].
2.3. Browning Intensities Measurements
For each sample, a cuvette was filled with 1.5 ml of freshly prepared crude plant solution and the optical density was read at wavelength 410 nm by a spectrophotometer at intervals of 20 minutes for 100 minutes. The absorbance was registered as the browning intensity and relative percentage (%) of browning intensity was calculated [15].
2.4. Evaluation of PPO Action and the Influence of Substrate Concentration
The increase in the absorbance at 410 nm was used for determining the PPO activity. Catechol was used as a substrate in all the experiments. Different concentration of substrate (0.01, 0.03, and 0.05 M), at pH 7.0 and temperature 40°C were used. Each tube has 2.0 ml of the substrate solution, sodium phosphate buffer (0.9 ml) with pH 7.0, and crude plant extract (0.1 ml). The blank tube has only the substrate and the buffer. A change in the absorbance of 0.001 minute-1 was considered as one unit of PPO activity [16]. The kinetic parameters (Km and Vmax) were determined according to Line weaver–Burk plot [17].
 | Table 1: Illustrate the scientific names, common names, and the aerial part of the plant used in this study. [Click here to view] |
2.5. Influence of pH on PPO Activity
The leverage of pH on enzyme activity was inspected using two kinds of buffer solutions, sodium acetate (0.2 M) for pH range of 3.0–5.5 and phosphate buffer for pH 6.0–8.5, respectively. The optimum pH of the enzyme was estimated under the typical assess situations by deliberating activity in the existence of buffers at diverse pH values extending from 3.5 to 8.0 [18]. Reaction rates of the enzymes were assayed as described previously and relative activity (%) was calculated.
2.6. Impact of Temperature on PPO Action
The effectiveness of temperature on PPO activity was assayed under standard survey circumstances at different temperatures ambit from 25°C to 55°C, and the buffer was warmed to appropriate temperature prior to the evaluation. The reaction rates of these enzymes were assayed as described previously and the percentage of relative activity was calculated. The activation of energy (AE) of enzyme estimated based on the Arrhenius plot of enzyme activity (Log V) versus reciprocal of absolute temperature (K-1) [19].
2.7. Statistical Analysis
All information analysis was carried out by the Microsoft excel 2003. Every experiment during the trials was done in triplicates. The out comings were registered as mean ± standard deviation (SD).
3. RESULTS
A relationship between the browning intensity and time was shown in Figure 1. The highest browning intensity was found in P. apple, followed by pomegranate seed and cucumber. The leverage of pH on PPO activity was determined by measuring enzyme activity according to Mizobutsi et al. [18]. The pH profile of the PPO enzyme was found at Figure 2. The optimum activity of the enzyme was registered at pH 7.0 for P. apple and cucurbita, and at pH 6.0 for rind and seeds of the pomegranate. The PPO of cactus leaf and cucumber fruits was shown to have two peaks at pH 4.0 and 7.0.
The influence of temperature on PPO activity is depicted in Figure 3. The optimal temperature for PPO activity in these plants was around 40°C. At 30°C, the relative activity was ranging from 45% in the cactus leaf to 72% in cucumber fruit. The AE that obtained during this investigation was shown in Figure 4.
To investigate the kinetic constants, Km and Vmax of PPO, initial reaction rates at different catechol concentrations (0.01, 0.03, and 0.05 M) were calculated. According to Line weaver-Burk plot, 1/V values were plotted against 1/[S] values (Fig. 5) and kinetic parameters were calculated from the chart. The Km and Vmax rates of the enzyme were summarized in Table 2. The highest activity (Vmax) of PPO was seen in P. apple (0.189 μmol/minutes), while the pomegranate rind has the lowest (0.03 μmol/minutes) (Table 2). The ratio of Vmax/Km for the tested plants is illustrated in Table 2. Table 2 explains the affinity of the enzyme to the substrate. The correlation between the browning intensity and PPO activity was shown in Figure 6. A strong correlation between the browning intensity and PPO activity in all plant samples was found [Correlation coefficient (R) = 0.9485].
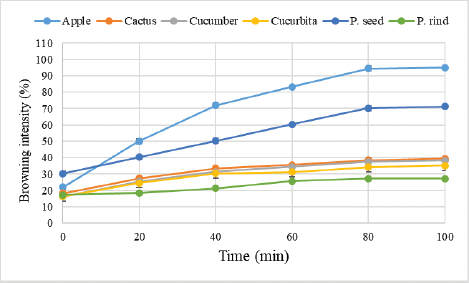 | Figure 1: Browning intensity (%) of P. apple fruits, pomegranate rind and seeds, cactus leaf, cucumber fruits, and cucurbita fruits with time (minute). Mean ± SD, n = 3. [Click here to view] |
 | Figure 2: Relative activity (%) of PPO in P. apple fruits, pomegranate rind and seeds, cactus leaf, cucumber fruits, and cucurbita fruits at different pH values. Mean ± SD, n = 3. [Click here to view] |
 | Figure 3: Relative activity (%) of PPO in P. apple fruits, pomegranate rind and seeds, cactus leaf, cucumber fruits, and cucurbita fruits at different temperature values. Mean ± SD, n = 3. [Click here to view] |
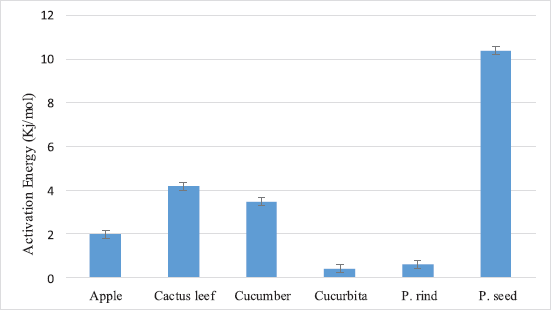 | Figure 4: The AE for PPOs extracted from P. apple fruits, cucumber fruits, cucurbita fruits, cactus leaf, pomegranate rind, and seeds. Mean ± SD, n = 3. [Click here to view] |
 | Figure 5: Determination of Km and Vmax values for PPOs from P. apple fruits, cucumber fruits, cucurbita fruits, cactus leaf, pomegranate rind, and seeds, using catechol as substrate. Mean ± SD, n = 3. [Click here to view] |
 | Table 2: Kinetic values for PPOs from different plant sources using catechol as substrate at 40°C and pH 7. [Click here to view] |
4. DISCUSSION
The PPO activity was boosted during 8–10 days of storing lead to early and quick browning [20]. The presence of oxygen, phenolic compounds, and PPO causes the color browning responses. Many fruits and vegetables are liable to enzymatic browning throughout handling and keeping [21]. The browning intensity in all tested plants was found to increase initially and became constant after about 80 minutes. Tissue's PPO level can fluctuate based on the growing environment and tissue ripeness. In potato, optimum pH was 5.0 for PPO enzyme [22], Longan fruit 7.0 [23]. The two optimum pH rates might have conducted due to the existence of isoenzymes. Our findings have similarities with the results obtained by [24, 25]. However, the variability with pH values based on the presence of specificity of the enzyme and its location influences the environmental effects.
 | Figure 6: The correlation between browning intensity (%) and PPO activity (μmol/minute). [Click here to view] |
The results of this study showed that the temperature causes highly effects on the enzyme activity (Fig. 3). Raising the temperature above 45°C caused the activity to decline drastically which may be due to the effect of denaturation. Optimum temperature is a necessary factor for picking of enzymes for manufacturing applications. Interestingly, most industrial enzymes have Vmax at 40°C–50°C [26]. The studied plants have an optimum temperature close to 40°C that means they can be used in industrial applications. Most PPOs extracted from studied plants have very low AE (Fig. 4), which is useful and required in the industrial processes to form the product. These results are following the results previously published were the browning intensity of P. apple exhibited the highest browning intensity and suggested that the PPO activity as the main factor in the browning reaction.
However, the ratio of Vmax/Km for the tested plants was calculated and from this ratio, the affinity of the enzyme to the substrate can be estimated. Therefore, P. apple had the highest Vmax/Km ratio, which means that the PPO of P. apple has the highest affinity for catechol as a substrate. The affinity of the enzyme in the studied plant was in the following order: P. apple > cucumber > pomegranate seed > cucurbita > cactus leaf > pomegranate rind (Table 2).
The strong correlation found between browning and PPO activity (Fig. 6) was in agreement with many previous studies [27], they are suggested that the PPO as one of the principal factors that affect browning in plants. These feedbacks confirmed that PPO was responsible for the color browning of the fruits. Therefore, fruits should be kept at sub-freezing temperatures for long storage [28].
5. CONCLUSION
During postharvest handling and stockpiling, fruits may be exposed to be injured which allows for enzymatic browning as a result of oxidation. This study showed that the apple fruit has the highest browning intensity and PPO activity compared to the tested plants. Moreover, PPOs of P. apple and cucurbita had its highest action at pH 7.0, while pomegranate rind and seeds at pH 6.0. Two optimum pH values were found for PPO of cactus and cucumber at pH 4.0 and 7.0 and this may indicate the presence of isoenzymes. The optimum temperature for PPO in all of the tested plants was around 40°C. Further studies should be carried out on these plants as a source of PPO for the degradation of phenols in wastewater to minimize water pollution in the future.
CONFLICT OF INTEREST
Authors declared that they do not have any conflicts of interest.
FINANCIAL SUPPORT
None.
REFERENCES
1. Queiroz C, Mendes MLM, Fialho E, Valente-Mesquita, VL. Polyphenol oxidase: characteristics and mechanisms of browning control. Food Rev Int 2008;24(4):361–75. CrossRef
2. Mariam A, Ahmed A, Insaaf A. Assessment of enzymatic browning and evaluation of antibrowning methods on dates. Int J Food Sci 2020;2020:1–9. CrossRef
3. Sukan A, Sargin S. Enzymatic removal of phenol from industrial wastewaters. J Biomater Nanobiotechnol 2013;4:300–7. CrossRef
4. Seo SY, Sharma VK, Sharma N. Mushroom tyrosinase: recent prospects. J Agric Food Chem 2003;51(10):2837–53. CrossRef
5. Liu Zhu, M. Characterization of polyphenol oxidase from the mustard tuber. J Hubei Univ 2005;4:625–712.
6. Simsek S, Yemenicioglu A. Partial purification and kinetic characterization of mushroom stem polyphenol oxidase and determination of its storage stability in different lyophilized forms. Process Biochem 2007;6(2):18–25. CrossRef
7. Fujita S, Yun-Zhen H, Tomoko M. Purification and characterization of polyphenol oxidase from edible yam (Dioscorea opposite). J Food Sci Technol Res 2006;12:235–9. CrossRef
8. Quesnel VC, Jugmohunsingh K. Browning reaction in drying cacao. J Sci Food Agric 2006;21(10):537–41. CrossRef
9. Raj SN, Sarosh BR, Shetty HS. Induction and accumulation of polyphenol oxidase activities as implicated in the development of resistance against pearl millet downy mildew disease. Funct Plant Biol 2006;33:563–71. CrossRef
10. Stalikas CD. Review: extraction, separation, and detection methods for phenolic acids and flavonoids. J Agric Food Chem 2007;51(10):253–67.
11. Gauillard F, Richard-Forget F. Polyphenol oxidases from Williams’s pear (Pyrus communis L., cv. Williams): activation, purification, and some properties. J Sci Food Agric 1997;74:49–56. CrossRef
12. Rapeanu G, Van Loey A, Smout C, Hendrickx M. Biochemical characterization and process stability of polyphenol oxidase extracted from Victoria grape (Vitis vinifera ssp. Sativa). Food Chem 2006;94:253–61. CrossRef
13. Çam M, ?çyer NC. Phenolic of pomegranate peels: extraction optimization by central composite design and alpha-glucosidase inhibition potentials. J Food Sci Technol 2015;52(3):1489–97. CrossRef
14. Aqil F, Munagala R, Vadhanam MV, Kausar H, Jeyabalan J, Schultz DJ, et al. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res Int 2012;49:345–53. CrossRef
15. Atrooz OM. Some properties of the polyphenol oxidase from Cyclamen persicum. Bull Fac Agric Cairo Univ 2009;60:408–14.
16. Mustafa UU, Aysun S, Kemal S. Characterization of sultaniye grape (Vitis vinifera L. cv. Sultana) polyphenol oxidase. Int J Food Sci Technol 2007;1:1–5.
17. Line weaver H, Burk D. The determination of enzyme dissociation constants. J Amer Chem Soc 1934;56:658–66. CrossRef
18. Mizobutsi GP, Finger FL, Ribeiro RA, Puschmann R, de Melo Neves LL, da Mota, WF. Effect of pH and temperature on peroxidase and polyphenol oxidase activities of litchi pericarp. Sci Agric 2010;67(2):213–7. CrossRef
19. Ibrahim MA, Olatominwa JO, Aliyu AB, Bashir M, Sallau AB. Partial characterization of protease from the leaves of jatropha curcas. Int J Biol 2012;4(4):79–85. CrossRef
20. Shelke MR, Fargade SA, Darade RV. Studies on polyphenol oxidase in pomegranate (Punica granatum L.). Asian J Biosci 2014;9(2):224–6. CrossRef
21. He Q, Luo Y, Pei Chen P. Elucidation of the mechanism of enzymatic browning inhibition by sodium chlorite. Food Chem 2008;110:847–51. CrossRef
22. Cemal Kasnak. Effects of anti-browning treatments on the polyphenol oxidase and antioxidant activity of fresh-cut potatoes by using response surface methodology. Potato Res 2020;10:1–14. CrossRef
23. Wilasinee C, Vicha S, Kanda W, Rumphan K, Pitipong, T. Minimally of polyphenol oxidase activity and controlling of rotting and browning of longan fruits cv. DAW by SO2 treatment under cold storage conditions. Int J Agric Res 2009;4(11):349–61. CrossRef
24. Ioannis K, Aleksandar B, Annette R. Biochemical and structural characterization of tomato polyphenol oxidases provide novel insights into their substrate specificity. Sci Rep 2019;9:4022–35. CrossRef
25. Taki D, Fatih S, Cigdem B, Nahit G. Extraction and biological activity of total anthocyanins from sweet cherry cultivars as polyphenol oxidase inhibitors. J Food Agric Environ 2013;11(3&4):572–5.
26. Devi RS, Narayan S, Vani G, Shyamala Devi CS. Gastroprotective effect of Terminalia arjuna bark on diclofenac sodium-induced gastric ulcer. Chem Biol Interact 2007;167:71–83. CrossRef
27. Nguyen TBT, Ketsa S, van Doorn WG. Relationship between browning and the activities of polyphenol oxidase and phenylalanine ammonia-lyase in banana peel during low-temperature storage. Postharvest Biol Technol 2003;30:187–193. CrossRef
28. Taranto F, Pasqualone A, Mangini G, Tripodi P, Miazzi MM, Pavan S, et al. Polyphenol oxidases in crops: biochemical, physiological, and genetic aspects. Int J Mol Sci 2017;18:377. CrossRef