1. INTRODUCTION
Amino acids are organic nitrogenous compounds and they are building blocks of proteins. Without amino acids, there would be no enzyme, and without enzyme, there will be no life. The L-amino acids are part of protein synthesis and have metabolic activity. Amino acids are absorbed by the plants through the stomata when sprayed externally. It has been proved that application of amino acids influences the physiological activities of the plants. The role of amino acids in stimulating the growth and chemical constituents was studied in several plant species such as Catharanthus roseus L. [1], Philodendron erbescens [2], and Thuja orientalis [3].
Microbes are extensively studied due to their beneficial use and valuable products such as amino acids, enzymes, organic acids, carbohydrates, vitamins, and polysaccharides during primary and secondary metabolism [4]. Amino acids producing bacteria were used commercially since 1950s. Many genera of bacteria are capable of amino acids production, for example, Corynebacterium, Brevibacterium, Bacillus, Enterobacter, Mycobacterium, and Escherichia [5]. According to Ivana et al. [6], plant growth promoting activity of fungi Purpureocillium lilacinum supernatant was evidenced through the increase of the percentage of tomato seed germination from 71% to 85% after 48 h of application. He also proved that, after 21 days, after application of the crude supernatant of P. lilacinum, the growth of tomato plants was similar to those grown with fertilizer amendment. Similarly, Malik and Sindhu [7] reported that different isolates of Pseudomonas obtained from chickpea and green gram produced the well-known plant growth promoter, the indoleacetic acid in the range of 10.2–31.2 μg/ml. The production of phytohormones was also reported in other plant growth promoting rhizobacteria strains including Azotobacter chroococcum [8], Azospirillum [9], Rhizobium species [10], Bacillus polymyxa [11], Pseudomonas fluorescens [12], and Pseudomonas putida [13].
L-glutamic acid is an important amino acid having a great role in the medical field. The amino acid is also widely used in the food industry, pharmaceuticals, animal feedstuffs, cosmetics, and other products. Glutamate is used as a fuel by the brain and is used as a flavor enhancer in the food industry [14]. Spraying different concentrations of glutamic acid increased the yield of wheat plants. It also improves nitrogen, phosphorus, and potassium content of wheat grains compared with the untreated control plant [15].
The present study was undertaken to screen the agriculturally important microbes, namely Pseudomonas and Bacillus spp., for the production of L glutamic acid. Another aim of the present study was to check the growth stimulatory effects of L-glutamic acid containing filtrate of Pseudomonas and Bacillus spp. through foliar application for growth and yield improvement of brinjal crop.
2. MATERIALS AND METHODS
2.1. Microorganisms, Chemicals, and Seed Culture
Standard cultures of Pseudomonas and Bacillus spp. were used throughout this study which were obtained from the different sources such as P. fluorescens MTCC8904, Bacillus licheniformis MTCC9555, Bacillus amyloliquefaciens MTCC10439, and Bacillus pumilus MTCC7615 from the Microbial Type Culture Collection and Gene Bank, Chandigarh, Punjab. P. fluorescens RCOF2144, P. fluorescens RCOF111, and Pseudomonas striata RCOF153 were obtained from the Regional Center of Organic Farming, Nagpur, Maharashtra. B. polymyxa NCIM87 and Bacillus megaterium NCIM77 were obtained from the National Chemical Laboratory, Pune, Maharashtra. Similarly, P. fluorescens 128, P. fluorescens AAU, and Bacillus subtilis 19 were obtained from Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth, Dapoli, Maharashtra, Assam Agriculture University, Jorhat, Assam, and International Crop Research Institute for Semi-Arid Tropics (ICRISAT), Hyderabad, respectively, whereas, P. fluorescens NSPL07 and P. fluorescens NSPL78 were the local isolates. Each strain was maintained on its respective agar media: Pseudomonas on King’s B medium [16] and Bacillus on nutrient agar.
Reagents for cultivation such as peptone, beef extract, and sodium chloride were obtained from HIMEDIA laboratory, India. Unless otherwise mentioned, the slants were incubated at 30°C and then stored at 4°C, which were subcultured at every 4 weeks. To prepare seed culture, a loop of cells from fresh slants was inoculated into a 250-ml flask containing 100 ml nutrient broth medium. The flasks were incubated on a rotary shaker at 30°C, 120 rpm for 24 h, and used as a seed for submerged cultivation.
2.2. Shake-flask Fermentation
The standard seed inoculums 5% (v/v) were used for shake-flask cultures. Fourteen different flasks of production medium containing peptone 10 g/l, beef extract 10 g/l, and sodium chloride 5 g/l were prepared. Poured 100 ml medium into every 250 ml Borosil flask and sterilized at 15 lb pressure for 15 min. Further, the broth was cooled and seed inoculum of Pseudomonas and Bacillus species was inoculated in each flask separately. The cultures were incubated for 96 h at 37°C with shaking at 120 rpm. After incubation, cells were pelleted by centrifugation at 25,704 g for 15 min and resulting supernatants were stored at 20°C. Each supernatant was subjected for the analysis of L-glutamic acid and bioefficacy testing.
2.3. Analysis of L-Glutamic Acid
L-glutamic acid was estimated according to Beautler [17]. For the present experiment, the required enzymes were obtained from “Megazyme International, Ireland.” The concentration of L-glutamic acid was calculated as follows:
Where,
V =Final volume (ml)
MW =Molecular weight of L glutamic acid (g/mol)
ε =Extinction coefficient of iodonitrotetrazolium (INT)-formazan at 492 nm
=19900 (I × mol−l × cm−l)
d=Light path (cm)
v=Sample volume (ml)
It follows for L-glutamic acid:
2.4. Bioefficacy Testing
In a preliminary experiment, different bacterial filtrates containing 0–500 ppm L glutamic acid were tried for plant growth promotion and it was noticed that bacterial filtrate containing 200 ppm L glutamic acid improved the overall growth of the plants, significantly. Then, based on the preliminary data, bacterial filtrate having 200 ppm L-glutamic acid was finalized for the field trial. Out of fourteen, four bacterial culture supernatants were selected for bioefficacy testing based on the amount of L-glutamic acid production. Specifically, supernatant of Pseudomonas fluorescens NSPL07, Pseudomonas fluorescens 128, Pseudomonas striata RCOF153 and Bacillus amyloliquefaciens MTCC10439 were selected.
2.5. Experimental Procedure
The research trial was conducted in Agronomy Research Farm of Nirmal Seeds Pvt. Ltd, Jalgaon, Maharashtra, India, during Rabi season of 2017–2018 in a completely randomized block design consisting of seven treatments with three replications of each. The seeds were sown in raised nursery bed, and the seedlings were transplanted to the main field when they were 4 weeks old. The plot size was 3 × 3.6 m2 with spacing between row to row 75 cm and plant to plant distance was 60 cm. Nirmal research hybrid of brinjal “Nirmal- 627 (Sanjay)” was used for the experiment.
Well drain organic matter-rich soil was selected. Farmyard manure 10-15 tonne per hectare was applied at the time of soil preparation. Application of 150 kg nitrogen, 100 kg single superphosphate, and 50 kg muriate of potash was added per hectare. Half dose of nitrogen, a full dose of single super phosphate, and a full dose of Muriate of Potash were applied as a basal dose. Remaining dose of nitrogen was applied after 20 days of transplanting. All other recommended cultural practices and plant protection measures were adopted to raise a healthy crop. The plot was irrigated adequately.
Bacterial supernatant that was prepared by shake flask fermentation technique was analyzed for the content of L-glutamic acid. Followed by the analysis, supernatant was diluted sequentially using water and achieved 200 ± 5 ppm concentration of L-glutamic acid and then sprayed it on the brinjal crop as per the treatments are given below. Plant growth promoting effects of bacterial filtrates such as an increase in the number of primary branches, plant height, number of fruits per plant, and overall yield improvement were studied.
2.6. Treatment Details
T1: Control (untreated)
T2: Supernatant of P. fluorescens NSPL07
T3: Supernatant of P. fluorescens 128
T4: Supernatant of P. striata RCOF153
T5: Supernatant of B. amyloliquefaciens MTCC10439
T6: Pure solution of L glutamic acid (200 ± 5 ppm, sprayable concentration)
T7: Planofix 0.3 ml/L
Three foliar sprays of each treatment were given at 15 days interval.
2.7. Statistical Analysis
Data of plant growth experiments were subsequently analyzed by ANOVA followed by Duncan’s test using Web Agri Stat Package (WASP) software (http://icargoa.res.in/wasp/index.php). The critical difference at 0.05 level of significance was calculated for the observed values along with average and standard deviation.
3. RESULTS AND DISCUSSION
3.1. L glutamic Acid in Bacterial Filtrates
Amino acids are precursors or activators of phytohormones and growth substances [18]. L-glutamic acid is one of the major amino acids that is present in a wide variety of foods. Glutamic acids and glycine are fundamental metabolites in the process of chlorophyll synthesis. P. fluorescens, P. striata, and many Bacillus spp. are agriculturally important microbes. In the present study, six of the eight species of Pseudomonas and three of the six Bacillus species produced a significant level of L-glutamic acid. As compared to Bacillus species, P. fluorescens 128, P. fluorescens NSPL07, and P. striata RCOF153 were the best L-glutamic acid producers. The enzymatic method was chosen for the detection of L-glutamic acid in which L glutamic acid was oxidized by coenzyme nicotinamide-adenine dinucleotide (NAD+) in the presence of enzyme glutamate dehydrogenase which formed 2 oxoglutarate, reduce NADH and ammonium ions (NH4+). Then enzyme diaphorase catalyzed the further reaction, in which NADH reduces INT chloride (INT) to an INT-formazan product, and the amount of INT-formazan formed in this reaction is stoichiometric with the amount of L-glutamic acid in bacterial supernatant [17]. This assay method is very specific for the detection of L-glutamic acid. D-glutamic acid and L-glutamate do not react with these enzymes. The quantity of L-glutamic acid produced differed from one organism to other because a genetic potential of each strain is different from other as well as the ability of microbes to excrete glutamic acid is markedly affected by the composition of media and growth conditions such as pH and temperature. Singh et al. [19] reported that addition of amino acid tryptophan in the medium enhances the production of actinomycin V by Streptomyces triostinicus, and on the contrary, the same amino acid showed an inhibitory effect in the production of candicidin from S. griseus [20]. L-glutamic acid production by Pseudomonas and Bacillus spp. will further improve by optimizing the carbon, nitrogen sources, and growth conditions.
3.2. Effect of L-Glutamic Acid on Growth and Yield of Brinjal
Date presented in Tables 1 and 2 show that the application of bacterial filtrate containing L-glutamic acid had significant effects on growth parameters of brinjal crop. However, all growth parameters increased after the foliar spray of bacterial filtrate as compared to control. Maximum plant height, more number of fruits, and higher brinjal yield per plant were recorded in the treatments T2, T3, T4, T5, and T6. Maximum plant height was recorded in treatment T4 which was at par with treatment T2, T6, and T7 but significantly superior over control. The height of the brinjal plants was measured from the ground level of the main stem to the apical bud (leaf apex) with the meter scale at maturity of the crop. The plant height increased significantly with the increased crop growth period. The increased plant height might be due to glutamic acid and other amino acids present in the filtrate which might have acted as a nitrogen source and increased the growth of brinjal. Amino acids were taken up in the leaf tissues of brinjal as ammonia and consequently assimilated within tissues and resulted in an increase in the various growth parameters. Amino acids may affect the general metabolism and morphogenesis [21] and regulate plant growth. Thon et al. [22] pointed out that amino acids provide nitrogen to plant cells which can be taken by the cells more rapidly than inorganic nitrogen. The ameliorative effect of bacterial supernatant containing L-glutamic acid or amino acids might be linked to the observable increase in photosynthetic pigments as well as leaf numbers. Consequently, the efficiency of the photosynthetic apparatus was increased due to amino acid treatments, which in turn considerably increased the biosynthesis of osmotic solutes. Therefore, bacterial supernatant can directly or indirectly affect the physiological activities of the plant and improve growth.
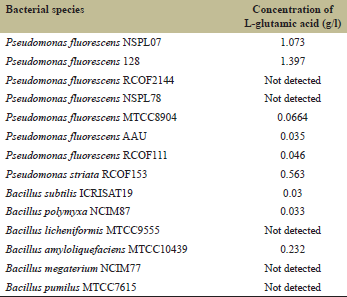 | Table 1: Content of L glutamic acid in bacterial filtrate (g/l) [Click here to view] |
The highest number of fruits per plant was recorded in treatments T7 and T3 which were at par with treatments T2, T4, and T5 but significantly higher as compared to untreated control (T1). The highest yield per plant was recorded in all the treatments from T2 to T7, and all the treatments were at par with each other but significantly superior over control. The number of fruits per plant was counted at the time of harvesting from first picking to last pickings. The weight of the fruits of the targeted plants was recorded at each picking, and the average yield of the fruits per plant was calculated. The phytotoxicity of supernatants also studied, and no phytotoxic symptoms were recorded in any of the treatment. Temporary whitening of the leaf was observed in the treatment of Planofix. Increased number of fruits per cluster due to the foliar spray of bacterial filtrates might be attributed to enhanced photosynthetic activity, resulting in increased production and accumulation of carbohydrates and favorable effect on vegetative growth and retention of flowers and fruits per cluster, which might have increased weight and number of fruits per cluster [23].
These results were compared with chemically synthesis L-glutamic acid solution (200 PPM) and untreated plants, for the growth and yield attributes of brinjal. Only L-glutamic acid was analyzed in bacterial filtrate, but bacterial secretion also contains other essential amino acids, some enzymes, and secondary metabolites [24]. Cumulative effects of multiple ingredients present in the filtrate of Pseudomonas along with glutamic acid might have improved the growth and yield of brinjal. According to the Mazher et al. [25], the highest values of all growth parameters such as a number of leaves, stem diameter, and number of roots were obtained with plants treated with 200 ppm glutamic acid. The present results are in agreement with Mazher et al. [25]. Amino acids can play vital roles in plants including acting as regulatory and signaling molecules. They are involved in the synthesis of other organic compounds, such as proteins, amines, alkaloids, vitamins, enzymes, terpenoids, and plant hormones that control various plant processes [26]. Amino acids also affect the synthesis and activity of some enzymes, gene expression, and redox homeostasis [27]. In this respect, Omer et al. [28] reported that foliar spray with amino acids improved the growth and chemical composition of the chamomile plant. The present results also indicated that the highest value of all growth parameters of brinjal crop was obtained with plants treated with bacterial filtrate containing 200 ppm L-glutamic acid.
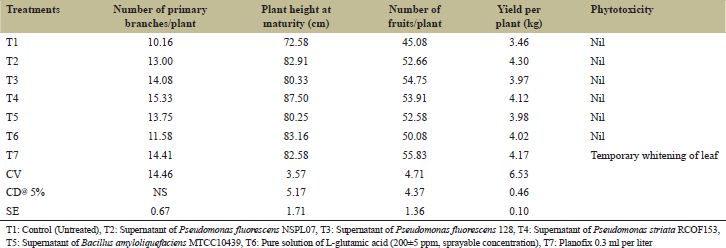 | Table 2: Effects of foliar application of bacterial filtrate containing L‑glutamic acid on growth and yield of brinjal [Click here to view] |
4. CONCLUSION
In the present study, P. fluorescens 128, P. fluorescens NSPL07, and P. striata RCOF153 were found to be the best L-glutamic acid producers, and from the bioefficacy point of view, it is preferable to spray bacterial filtrate or 200 ppm L-glutamic acid solution to enhance the growth and yield of brinjal.
5. REFERENCES
1. Iman MT, Bekheta MA, Mona H, Mahgoub M. Physiological response of periwinkle plants (Catharanthus roseus L.) to tryptophan and putrescine. Int J Agric Biol 2005;7:210-3.
2. Dahab TA, Aziz NG. Physiological effect of diphenylamine and tryptophan on the growth and chemical constituents of Philedendron erubescene plants. World J Agric Sci 2006;2:75-81.
3. Aziz NG, Azza AM, Farahat MM. Response of vegetative growth and chemical constituents of Thuja orientalis L. plant to foliar application of different amino acids at Nubaria. J Am Sci 2010;6:295-301.
4. Demain AL. Achievements in microbial technology. Biotechnol Adv 1990;8:291-301. CrossRef
5. Bona R, Moser A. Modeling of L-Glutamic acid production with Corynebacterium under biotin limitation. Chem Biochem Eng Q 1988;12:25-9.
6. Ivana AC, Juan MC, Sabrina SG, Jose MZ, Maria FL, Cavalitto SF. Plant growth promotion activity of keratinolytic fungi growing on a recalcitrant waste known as hair waste. Biotechnol Res Int 2015;2015:10.
7. Malik DK, Sindhu SS. Production of indole acetic acid by pseudomonas sp: Effect of coinoculation with Mesorhizobium sp. Cicer on nodulation and plant growth of chickpea (Cicer arietinum). Physiol Mol Biol Plants 2011;17:25-32. CrossRef
8. Muller F, Deigele C, Ziegler H. Hormonal interactions in the rhizosphere of maize (Zea mays L.) and their effects on plant development. J Pflanzen Bodenkd 1989;152:247-54. CrossRef
9. Bar T, Okon Y. Induction of indole-3-acetic acid synthesis and possible toxicity of tryptophan in Azospirillum brasilense spp7. Symbiosis 1992;13:191-8.
10. Hirsch AM, Fang Y. Plant hormones and nodulation: What’s the connection? Plant Mol Biol 1994;26:5-9. CrossRef
11. Holl FB, Chanway CP, Turkington R, Radley RA. Response of crested wheatgrass (Agropyron cristatum L.), perennial ryegrass (Lolium perenne) and white clover (Trifolium repens L.) to inoculation with Bacillus polymyxa. Soil Biol Biochem 1988;20:19-24. CrossRef
12. Dubeikovsky AN, Mordukhova EA, Kochetkov VV, Polikarpova FY, Boronin AM. Growth promotion of blackcurrant softwood cuttings by recombinant strain Pseudomonas fluorescens BSP53a synthesizing an increased amount of indole-3-acetic acid. Soil Biol Biochem 1993;25:1277-81. CrossRef
13. Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A, Weyens N, et al. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol 2009;75:748-57. CrossRef
14. Garattini S. Glutamic acid, twenty years later. J Nutr 2000;130:901S-9S. CrossRef
15. Allah MM, EI-Bassiouny HA, Bakry BA, Sadak MS. Effect of arbuscular mycorrhiza and glutamic acid on growth, yield, some chemical composition and nutritional quality of wheat plant grown in newly reclaimed sandy soil. Res J Pharm Biol Chem Sci 2015;6:1038-54.
16. King EO, Ward MK, Raney DE. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 1954;44:301-7.
17. Beautler HO. L-Glutamate, colorimetric method with glutamate dehydrogenase and diaphorase. In: Bergmeyer HH, editor. Methods of Enzymatic Analysis. 3rd ed., Vol. 8. Cambridge, UK: VCH Publishers (UK) Ltd.; 1990. p. 369-76.
18. Gros JA. Amino Acids Synthesis and Metabolism Physiology of Plants and Their Cell. New York, Sydney, Braunschweig: Pergamon Press Inc.; 1973. p. 202.
19. Singh V, Khan M, Khan S, Tripathi CK. Optimization of actinomycin V production by Streptomyces triostinicus using artificial neural network and genetic algorithm. Appl Microbiol Biotechnol 2009;82:379-85. CrossRef
20. Sanchez S, Demain AL. Metabolic regulation of fermentation processes. Enzyme Microb Technol 2002;31:895-906. CrossRef
21. Basu A, Sethi U, Mukherjee SP. Regulation of cell proliferation and morphogenesis by amino acids in Brassica tissue cultures and its correlation with threonine deaminase. Plant Cell Rep 1989;8:333-5. CrossRef
22. Thon M, Maretzki A, Korner E, Soki WS. Nutrient uptake and accumulation by sugar cane cell culture in relation to growth cycle. Plant Cell Tissue Organ Cult 1981;1:3-14. CrossRef
23. Mohsen K. Effects of Zn, Fe and their combination treatments on the growth and yield of tomato. Bull Environ Pharmacol Life Sci 2013;3:109-14.
24. Pankaj K, Ramesh CD, Dinesh KM, Yong-Ha P, Vivek KB. Isolation of plant growth-promoting Pseudomonas spp. PPR8 from the rhizosphere of Phaseolus vulgaris L. Arch Biol Sci 2016;68:363-74. CrossRef
25. Mazher AA, Zaghloul SM, Mahmoud SA, Siam HS. Stimulatory effect of kinetin, ascorbic acid and glutamic acid on growth and chemical constituents of Codiaeum variegatum L. Plants. Am Eurasian J Agric Environ Sci 2011;10:318-23.
26. Glawischnig E, Tomas A, Eisenreich W, Spiteller P, Bacher A, Gierl A. Auxin biosynthesis in maize kernels. Plant Physiol 2000;12:1109-20. CrossRef
27. Rai VK. Role of amino acids in plant responses to stresses. Biol Plant 2002;45:481-7. CrossRef
28. Omer EA, Said-Al Ahl HA, El Gendy AG, Shaban KH, Hussein MS. Effect of amino acids application on production, volatile oil and chemical composition of chamomile cultivated in saline soil at Sinai. J Appl Sci Res 2013;9:3006-21.