The rapid increase in the global population has provided much impetus to major sources of energy supply, such as coal, petroleum, and natural gas. Due to the inclination toward increase demand for petroleum-derived fuel, a huge amount of petroleum waste in the form of sludge is generated. The disposal processes employed are very cost bearing involving expensive physico-chemical methods [1]. Hence, biodegradation of the sludge waste using biological organisms is the need of the hour. In this context, cyanobacteria could play a pivotal role in biodegradation as it has marked its presence more than 3 billion years ago and are believed to make their habitat in fresh to marine water bodies, but their dominant presence as primary producers in extremely arid, salt lakes and hot springs, and the hot and cold desserts are significant [2,3]. Cyano in the name cyanobacteria got derived due to the presence of the blue color pigment phycocyanin (PC) combined with the green chlorophyll pigment gives rise to the colloquial label blue-green algae [3]. Because they are photoautotrophs and have the ability to fix nitrogen from the atmosphere, cyanobacteria are more resilient in contaminated environments [4]. These organisms have a strong capability for creating copious amounts of effective compounds to guard against oxidative and radical stressors due to their phototrophic lifestyle and frequent exposure to high oxygen and radical stresses [5]. Cyanobacteria by virtue of their vast gene pool and plasticity are stated as potent tools for environmental rehabilitation [6]. Therefore, through either biodegradation or bioaccumulation, cyanobacteria are preferred for the bioremediation of environmental contaminants. Efficient absorption of organic matter facilitates cyanobacteria to be capable of biodegradation, transformation, and biosorption of industrial wastewater pollutants [7]. Products and the petroleum having composition of polyaromatic hydrocarbons (PAHs), mono-, and bi-aromatic compounds, alkanes, asphaltenes, resins, are investigated to be bioremediated by cyanobacterial organisms such as Oscillatoria, Spirulina, Synechocystis, to mitigate the toxicological impact on the ecosystem [8,9]. Cultures of blue-green algae, Synechococcus spp., axenic and in consortium with Chlorella vulgaris, remediate kerosene on cultivation [1].
Along with being the producers of different cellular secondary metabolites and biofuels, cyanobacteria are deemed to be beneficial organisms for waste treatment. These blue-green algae have been as well reported to bear a symbiotic relationship with other aerobic or anaerobic organisms in the natural environment, exhibiting hydrocarbon degradation present in oil. Cyanobacteria have rapid metal removal kinetics and the ability to treat multiple metal-containing molecules [4,7]. Through an elongated recovery duration, cyanobacteria Nostoc muscorum and Anabaena spp. displayed bioaccumulation to mitigate the deleterious impact of the forms of arsenic, AsV and AsIII [10]. It has been estimated that approximately 28220 tons of sludge are generated in Indian and globally 3 million tons of sludge are generated in China through the exploration by various Chinese refinery companies [11,12]. Beyond that, the composition of these oily sludge is made of compounds and heavy metals as phenol (90–100 mg/kg), chromium (27–80 mg/kg), nickel (17–25 mg/kg), manganese (19–24 mg/kg), zinc (7–80 mg/kg), copper (32–120 mg/kg), cadmium (0.8–2 mg/kg), and lead (0.001–0.12 mg/kg) [7] which are codominantly lethal for disposal in the environment directly before treatment. The hazardous impact of these compounds on lives existing on land or in water bodies (micro-macro organisms) does not remain hidden or unnoticed. Discharge of untreated oil sludge directly to the environment constitutes a peril to the life forms residing in it along with the promotion of unavailability of fresh waters and unpolluted soil. Thus, this demands for holistic understanding and action for characterization, management, and safe and organized disposal of these oil wastes. A treatment before discharge to land or the natural water bodies is a crucial requirement. It shall be an innovative approach for these three specific cyanobacterial organisms to be investigated for the oil refinery-generated sludge through revealing their functional enzyme activities contributing to the bioremediation. The Nostoc spp. being yet to be reported for their remediation function toward oil tank sludge, its biodegradation mechanism is quantifiable through the gas chromatography techniques.
Among the plenty of physical and chemical methods and approaches that are expensive, resources dependent, sources of secondary pollutants, laborious, and inefficient for large-scale application, bioremediation comes across as the safest and most suitable solution. The petroleum hydrocarbons are among the most persistent organic pollutants and its removal from the environment is accomplished by microorganism performed degradation [12]. And in that regard, features like being productive due to the ability of nitrogen fixation, inexpensive, natural resistance acquired against environmental pollutants make these cyanobacteria ideal and advantageous over alternative microorganisms for this purpose.
2. MATERIALS AND METHODS
2.1. Source Location of Treatment Samples
The treatment sample, tank bottom oil sludge, was sourced from the Numaligarh Refinery Limited (NRL) (26.5786° N, 93.7848° E) plant, District Golaghat, Numaligarh, Assam.
2.2. Culture Conditions of Cyanobacterial Samples
Three routinely maintained axenic cyanobacterial cultures of N. muscorum, Anabaena variabilis, and Nostoc spp. in BG11o medium were selected for the present investigation. Culture conditions include an air-conditioned chamber at 24 ±2°C with a photon fluence rate of 50 μmol m−2 s−1 and control illumination of 12 h light and 12 h dark was maintained [13]. The cultures has been purified morphologically and identified through their 16S ribosomal RNA sequence [14] submitted to the gene bank to obtain their accession numbers.
2.3. Microscopical Analysis
The morphology of the treated as well as the untreated cultures was studied using the Radical RXL-4B light microscope with an equipped digital camera under 45×. Features such as the cell shape and size, plane of division, dimensions, color, motility, and sheath were studied in addition to the special feature of heterocyst formation and presence of akinetes, necridiabaecytes to resolve a comparative study between the untreated and sludge-treated cyanobacterial cultures.
2.4. Establishment of Sludge Lethal Dose Concentration
Strains of A. variabilis, N. muscorum, and Nostoc spp. were cultivated in BG-11o medium individually to obtain cells in their exponential growth phase. The sludge sample was prepared for inoculation by dissolving it in n-hexane in a 1:1 ratio. To determine the sludge lethal dose concentration, portions were drawn from the cyanobacterial mother cultures and inoculated with a graded range of concentrations of tank bottom sludge (0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 20, 30, 40, and 50 mg/mL−1) to culture individually [15]. The culture’s optical densities were measured at 663 nm on the 28th day of inoculation to define the sludge lethal dose concentrations.
2.5. Growth Assessment of Cyanobacterial Cultures
2.5.1. Chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoids
Assessment of the cyanobacterial growth was conducted based on the cultures Chl a, Chl b, and carotenoids content with few modifications [16]. The treated and untreated culture suspensions were prepared for the extraction by centrifuging for 10 min at 10,000 rpm. The pellets obtained were dissolved in methanol in 10 mL, heated in water bath for 30 min, and incubated overnight for the complete extraction of the pigments. The solution was vortexed and centrifuged for 10 min at 10,000 rpm and measured for absorbance by a spectrophotometer. The readings were taken at respective wavelengths specific to chlorophyll a, chlorophyll b, and carotenoids to calculate the pigment content using the following formulae [17],
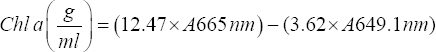 |
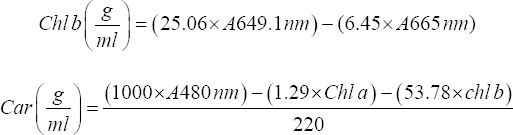 |
PC = Phycocyanin, PE = Phycoerythrin, APC = Allophycocyanin
2.5.2. Phycobilisomes
The phycobilisome pigment content including phycoerythrin (PE), PC, and allophycocyanin (APC) was prepared by centrifuging the culture suspension at 4000 rpm, thereby washing the pellet with 1M phosphate buffer [18]. The extraction was conducted by giving a 30 min water bath to the pellets dissolved in methanol. The vortexed solution was centrifuged to obtain the supernatant containing the extracted pigment and its absorbance was measured at respective wavelengths using a spectrophotometer and using the following formulae, the pigments were quantified.
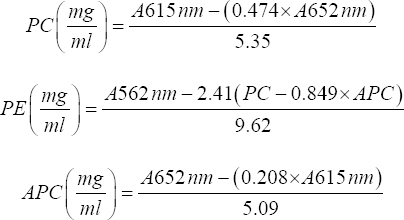 |
PC = Phycocyanin, PE = Phycoerythrin, APC = Allophycocyanin
2.6. Hydrocarbon Biodegradation and Degradation Rate
The sample preparation for the determination of total petroleum hydrocarbon (TPH) content of the treated and untreated sludge was conducted [19], sketched protocol including few modifications. Using HPLC-grade hexane in a ratio of 1:1, the samples were extracted with centrifugation at 15000 rpm for 5 min. The obtained pellets were sonicated using a titanium probe of 6 mm diameter of an ultrasonic processor (Rivotek, India) for 90 min with 5 s on and 2 s off intervals. The TPH accumulated solvent was kept resting in a separating funnel to obtain segregated layers with the process being repeated twice to obtain the residual TPH. The complete hydrocarbon was extracted using a rotary evaporator (IKA, Germany). Gas chromatography (GC) was performed to measure the TPH of the prepared samples with an equipped Flame-Ionization Detector (FID) (Thermofisher TRACE 1600 Series). The used column specifications were TG-WAXMS GC COL capillary column with dimensions of 30 m × 250 mm × 0.25 μm. The analysis was conducted for a total of 50 min run time in a constant flow rate of 1 mL/min of hydrogen carrier gas in a split-less mode. 230°C inlet temperatures, 250°C detector temperatures, and 190°C oven temperature were maintained to run the injected 2 μL samples. Analysis, peak integration, and the required refining procedure were performed in the Chromeleon 7.2/7.3 software for GC SE (single user, single TF instrument license).
The sludge degradation (S) was calculated by the following formula:
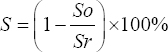 |
Where So = Sludge concentration in control and Sr = Sludge concentration in treatment culture.
2.7. Sludge Degradation Kinetics
The kinetics of the degradation reactions were measured based on rate laws to determine the order of the reaction.
 |
 |
 |
Where k = rate constant, [A] = initial sludge concentration, [A]o = sludge concentration relative to time t and n = order of reaction.
2.8. Enzyme Assays
2.8.1. Preparation of enzyme extracts
The extraction of enzymes from sludge treated as well as positive control cultures of cyanobacteria devoid of sludge was performed with slight modifications [19] by homogenizing in ice-cold 50 mM phosphate buffer and centrifuged at 4°C at 15000 rpm. Freshly prepared cell lysis buffer was added to the obtained pellets and centrifuged to yield a cell-free supernatant. The extract obtained was subjected to 60% ammonium sulfate precipitation and suspended in 50 mM phosphate buffer. Furthermore, purification of the extracts was undertaken for dialysis with phosphate buffer and changed at 3 h intervals for 12 h. This purified and concentrated enzyme extract was then assayed for polyphenol oxidase (PPO), catalase, lipase, esterase, and dehydrogenase enzyme activity.
2.8.2. Measurement of PPO activity
The PPO activity was performed to obtain a standard curve using a concentration range of 0.05 mg/mL to 0.25 mg/mL of a sample [20]. The PPO assay included an enzyme cofactor, a suitable buffer, and PPO enzyme solution. For the determination of the activity of the extracted enzyme, the reaction components were repeated with 100 μL of the enzyme extract instead of the known PPO solution. The reaction contents were incubated at 40°C for 20 min. The obtained absorbance values at 370 nm were plotted on the obtained standard curve and the activity was determined.
2.8.3. Catalase activity
Activity of catalase [EC 1.11.1.6] in the sludge-treated and untreated cyanobacterial cultures was determined by the addition of reaction contents [21] containing definite enzyme extract, 1% Triton X-100, followed by 30% hydrogen peroxide. The activity was supposed to be measured with reference to the height of the effervescence formed in the reaction tube. On the contrary, because the activity was minimal, the presence or absence of the effervescence was used to conclude with the catalase activity as qualitative data.
2.8.4. Lipase activity
Lipase [EC 3.1.1.3] activity was performed by determining the absorbance of the reaction mixture containing lipase solution and Tween 20 in phosphate buffer used as the substrate was taken at 450nm after incubating it for 10 min [22]. The lipase activity of the enzyme extract was determined with the aid of a calibration curve obtained between a concentration range from 0 mg/mL to 4 mg/mL for the mentioned reaction setting with slight increase in the reaction volumes as a modification of the original protocol.
2.8.5. Esterase activity
Esterase [EC 3.1.1.8] activity was determined with slight modifications [23] by adding sodium phosphate buffer and p-nitrophenol acetate in methanol to 1 mL of the enzyme extract and incubated for 10 min at 55°C, followed by measurement of absorbance at 347 nm.
2.8.6. Dehydrogenase activity
Activity of the dehydrogenase [EC 1.1.1.1] enzyme was determined [24] through a reaction mixture containing a buffer substrate solution that is prepared by mixing 4.8 mL of 60% sodium lactate solution (Fisher) and 95.2 mL of 0.1 M Tris buffer (pH 9.0) and diphosphopyridine nucleotide along with the substrate iodonitrotetrazolium chloride dye and gelatine and incubated for 30 min at 37°C. The phenazine methosulfate and the enzyme extract were added to the incubated mixture and kept at 37°C for 15 min. The reaction was stopped by the addition of 0.35M HCl and the absorbance was measured at 540 nm by a spectrophotometer. The activity of dehydrogenase was calculated using a standard curve.
2.9. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
The sample obtained by processing the oil sludge sample treated by the Nostoc spp. culture through centrifuging, followed by sonicating the pellet in an organic solvent and concentrating through rotary evaporation, has been taken for GC-MS analysis. The biodegradation indices were calculated by the following equation,
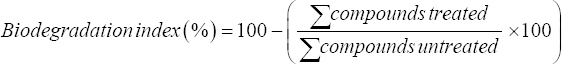 |
2.10. Statistical Analysis
The experiments generated data were evaluated by one-way analysis of variance with the Tukey-Kramer procedure and the data were considered to be significant with P < 0.05.
3. RESULTS
3.1. Morphological Characteristics
The morphological characteristics of the three axenic cultures N. muscorum, A. variabilis, and Nostoc spp. are depicted in Figure 1. These cultures were identified from various habitats and are maintained in the laboratory. The cultures have been identified using molecular phylogeny and accession no have been obtained from the gene bank [14] - N. muscorum (KR709122), A. variabilis (KR709104), and Nostoc spp. (KR709128), respectively.
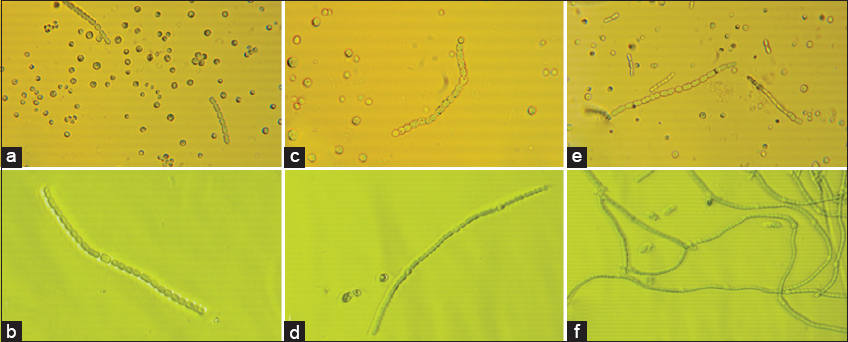 | Figure 1: Micrograph of pure cultures and tank bottom sludge treating cultures. (a) pure Nostocmuscorum, (b) Nostoc muscorum treating tank bottom sludge, (c) pure Anabaenavariabilis, (d) Anabaena variabilis treating tank bottom sludge, (e) pure Nostoc spp., and (f) Nostoc spp. cultures treating tank bottom sludge. [Click here to view] |
The sludge treated cultures upon examining with their untreated cultures, N. muscorum showed barrel-shaped unbranched filamentous structure indicating to be in the seriate stage, with cell size about 6±0.38 μm in length [Figure 1a, 1b], A. variabilis exhibited filamentous vegetative cells in the size range of 5.1 ± 0.27 μm in length, akinetes, and rounded terminal cells and initially devoid of heterocyst’s [Figure 1c, 1d] and lastly Nostoc spp. exhibited tapered terminal and barrel-shaped filamentous vegetative cells, about 6.3 ±0.18 μm long [Figure 1e, 1f]. The treated cultures’ morphology has not been changes as compared with the untreated cultures [Figure 1a, c, and e] and it confirms that the cell machinery has been functioning in normal way when subjected to sludge stress.
3.2. Establishment of Lethal Dose Concentration
The graph presents the relationship between sludge concentration (mg/mL) and Chl a content in three cyanobacterial species: N. muscorum, A. variabilis, and Nostoc spp. [Figure 2]. Chl a is widely used as an important growth parameter in cyanobacteria to determine the threshold concentration at which the sludge treatment could retain the growth parameters of the cell. All cultures showed an initial increase in Chl a concentration with increasing sludge concentration with maximum sludge retention at 7–9 mg/mL. Consequently, beyond 9 mg/mL higher concentrations of the sludge utilization probably decline, reducing their ability to photosynthesize and survive, which is crucial for bioremediation potential. Hence, 9 mg/mL is used as a minimum inhibitory threshold for all experiments that are investigated in the present study.
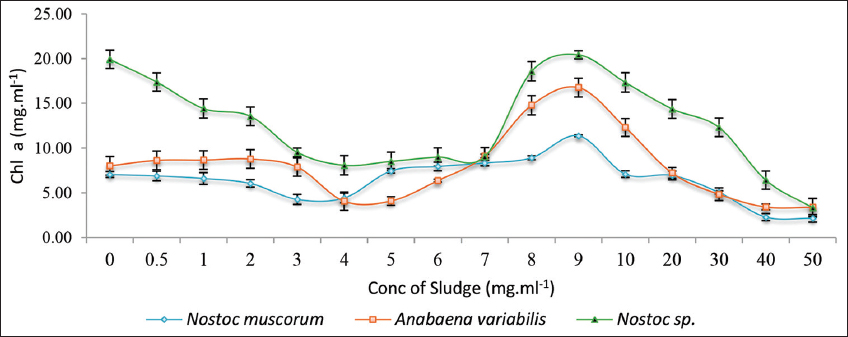 | Figure 2: A graphical representation of the lethal dose concentration of the tank bottom sludge on the three treated cyanobacterial cultures on the 28th day of incubation. Individual cultures were duplicated with the respective ± standard deviation values and P < 0.05. [Click here to view] |
3.3. Growth Parameters
3.3.1. Chl a, Chl b, and carotenoids
The Chl a content of N. muscorum, A. variabilis, and Nostocspp. was monitored over 28 days with 9 mg/mL sludge concentration [Figure 3]. Initially, all strains exhibited a higher Chl a concentration, which gradually declined within the first 4 days but subsequently stabilized with a sluggish recovery trend. Nostoc spp. exhibited the highest Chl a levels throughout, followed by A. variabilis, while N. muscorum showed the lowest. After 28 days, the growth was shunted, indicating a gradual adaptation and possible acclimatization to environmental conditions.
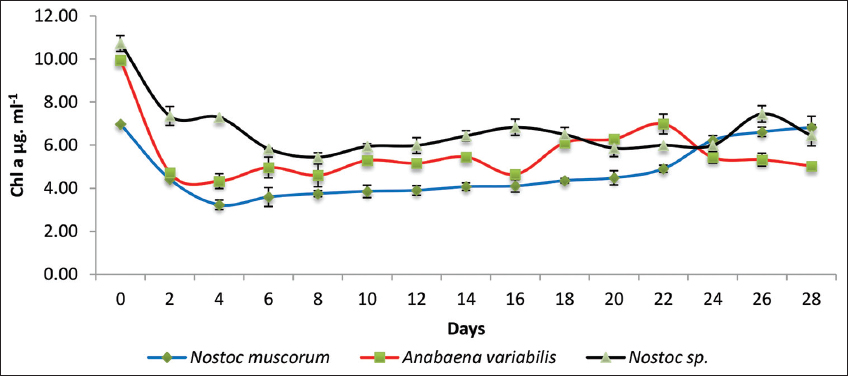 | Figure 3: A graphical representation of the chlorophyll a concentration of the three cyanobacterial cultures treated with tank bottom sludge on the 28th day of incubation. Individual cultures were duplicated with the respective ± standard deviation values and P < 0.05. [Click here to view] |
Similar trends in context to Chl b were observed for the three cultures under study with sludge concentration of 9 mg/mL incubated for 28 days [Figure 4]. N. muscorum exhibits frequent fluctuations with Chl b concentration and in contrast, A. variabilis showed consistently low values. Furthermore, the analysis suggests that Nostoc spp. may have a higher adaptability or resilience, making it a better candidate for applications requiring sustained chlorophyll retention in cell functionaries.
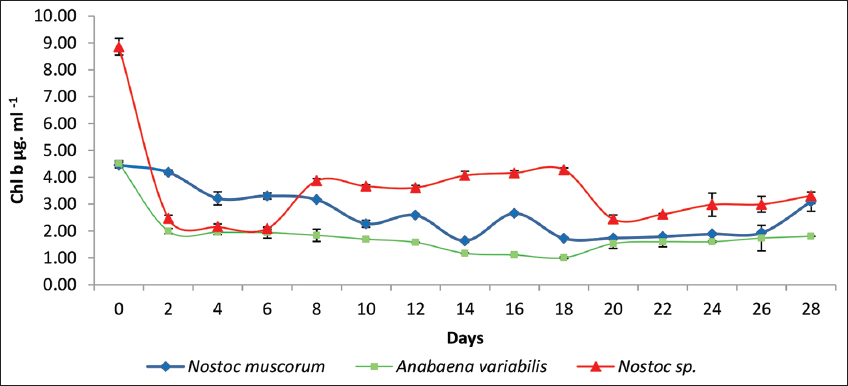 | Figure 4: A graphical representation of the chlorophyll b concentration of the three cyanobacterial cultures treated with tank bottom sludge on the 28th day of incubation. Individual cultures were duplicated with the respective ± standard deviation values and P < 0.05. [Click here to view] |
The carotenoid content in response to the sludge treatment of 9 mg/mL exhibited similar results as Chl a and Chl b growth patterns [Figure 5]. Initially, Nostoc spp. unveiled the highest carotenoid concentration of 4.5 μg/mL, followed by a sharp decrease within the first 4 days and then consistent carotenoid content at lower levels. In contrast, N. muscorum and A. variabilis displayed lower carotenoid levels of μg/mL and also showed a similar decreasing trend before stabilizing. All the three cultures maintained relatively low carotenoid content, ranging from 0.5 to 1.0 μg/mL with minor fluctuations between day 6 and 18, but a gradual increase was observed on day 20 with Nostoc spp. cultures exhibiting higher catenoid concentrations, suggesting a better adaptation upon stress, followed by recovery with increased concentrations.
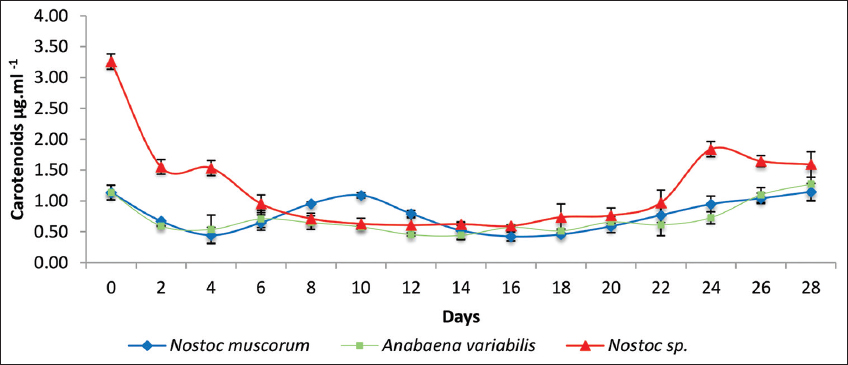 | Figure 5: A graphical representation of the carotenoids concentration of the three cyanobacterial cultures treated with tank bottom sludge on the 28th day of incubation. Individual cultures were duplicated with the respective ± standard deviation values and P < 0.05. [Click here to view] |
The overall pattern of the growth characteristics by three cultures suggests that Nostoc spp. as a promising candidate for further applications in pigment augmentation and oxidative stress resistance in response to sludge studies.
3.3.2. Phycobillisomes
The phycobilisomes content of cultures under study in the form of PC, PE, and APC in response to sludge treatment showed relatively lower concentrations of phycobiliproteins compared to their respective controls [Figure 6]. Among the cultures, A. variabilis demonstrates a moderate increase in all three pigments, followed by N. muscorum and Nostoc spp., exhibiting similar trends. The results further suggest that the control conditions favored the accumulation of phycobiliproteins, particularly APC, while the experimental cultures show lower but consistent production of these pigments. The differences observed could be attributed to variations in the metabolic activity, growth conditions, or genetic potential of the respective cyanobacterial strains in the sludge stress environment. These findings also indicate that these cyanobacterial cultures may require a longer period to adapt to the initial toxicity of the sludge, as all the pigments after the initial drop did take up a leap, and that with a longer incubation period, if provided, the cultures may exceed the initial pigment content.
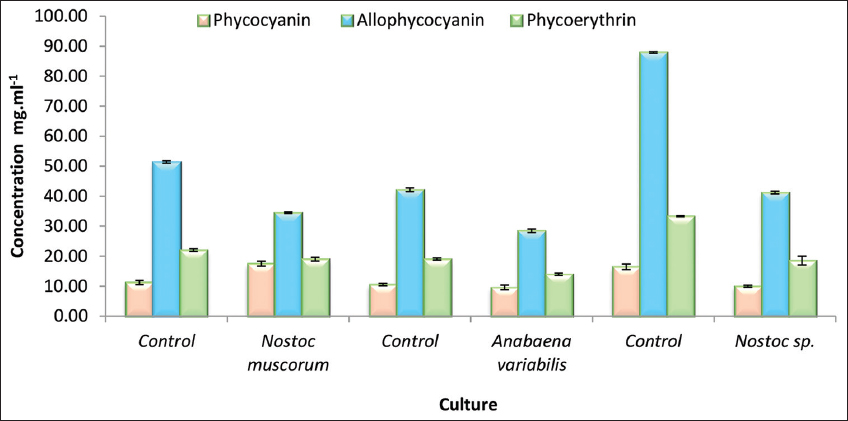 | Figure 6: A graphical representation of the phycobilisomes protein concentration of the three cyanobacterial cultures treated with tank bottom sludge on the 28th day of incubation. Individual cultures were duplicated with the respective ± standard deviation values and P < 0.05. [Click here to view] |
3.4. Biodegradation Potential of the Cyanobacterium by Virtue of the Degradation Rates
The composition of the tank bottom sludge from the GC-FID output was found to be of alkanes, isomeric alkanes, alkenes, cycloalkenes, and aliphatic and aromatic hydrocarbons. The pre-incubation TPH content of the untreated sludge was 495.63 g/kg with a spectrum of hydrocarbons ranging from C3 to C22 illustrated in Figure 7. The residual TPH content of the sludge on the 28th day after incubation with three cyanobacterial cultures was obtained to be 29.01 g/kg chla.mg/mL for N. muscorum, 0.57 g/kg chla.mg/mL for A. variabilis, and 0.62 g/kg chla.mg/mL for Nostoc spp., respectively. Subsequently, N. muscorum exhibited a TPH reduction of 94.14% followed by A. variabilis with reduction of 99.88%, and Nostoc spp. with 99.87% TPH reduction [Table 1]. Evidently, the strain of Nostoc spp. performed most efficiently with a reduced hydrocarbon spectrum of C3–C12, followed by A. variabilis with C3–C12 but a slightly higher total peak area than the former and N. muscorum with C3–C22 with a highly reduced total peak area from that of the untreated sludge sample. Thus, in addition to these numerical figures, the reduced peak area in the obtained GC-FID chromatogram further contributes to the inference that these cyanobacterial strains are significantly capable of biodegrading the long-chain hydrocarbons present in the tank bottom sludge waste. This describes the potential of these cyanobacterial strains to bioremediate these refinery-generated tank oil sludge, as exhibited in the mentioned frame of incubation, on upscaling. The TPH reduction was further investigated for biodegradation kinetics for all three sludge-treated cyanobacterial cultures [Figure 8], which demonstrated a first-order degradation kinetic model, where the TPH concentration decreases exponentially over time. This suggests that the rate of hydrocarbon degradation is proportional to the remaining TPH concentration as depicted from the eq 1, 2, and 3 which also confirms that the steeper degradation curve results in the higher rate constant (k).
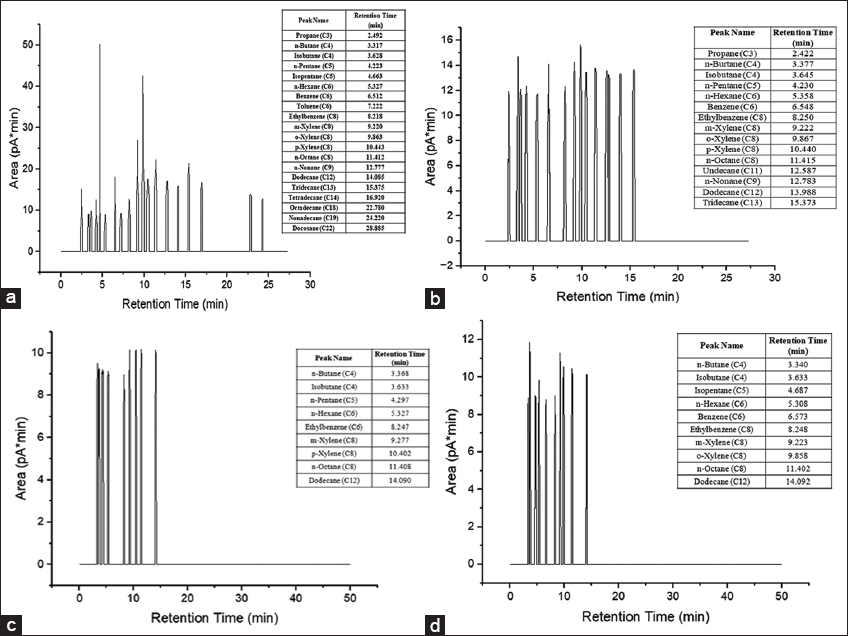 | Figure 7: Gas chromatography flame ionization detection chromatogram representing the total petroleum hydrocarbon of (a) Sludge; (b) sludge treated Nostoc muscorum; (c) sludge treated Anabaena variabilis; (d) sludge-treated Nostoc spp. on the 28th day of incubation [Click here to view] |
Table 1: The reaction rate per cent on the 28th day, reaction kinetic equation, and their respective determination coefficient values by the three treatment cultures, having “y” as the total petroleum hydrocarbon concentration.
| Culture | Sludge degradation rate % | Kinetic equation | R2 |
|---|---|---|---|
| Nostoc muscorum | 94.14 | y = −2368.x + 94486 | 0.99 |
| Anabaena variabilis | 99.88 | y = −3094.x + 91226 | 0.993 |
| Nostoc spp. | 99.87 | y = −3134.x + 92520 | 0.99 |
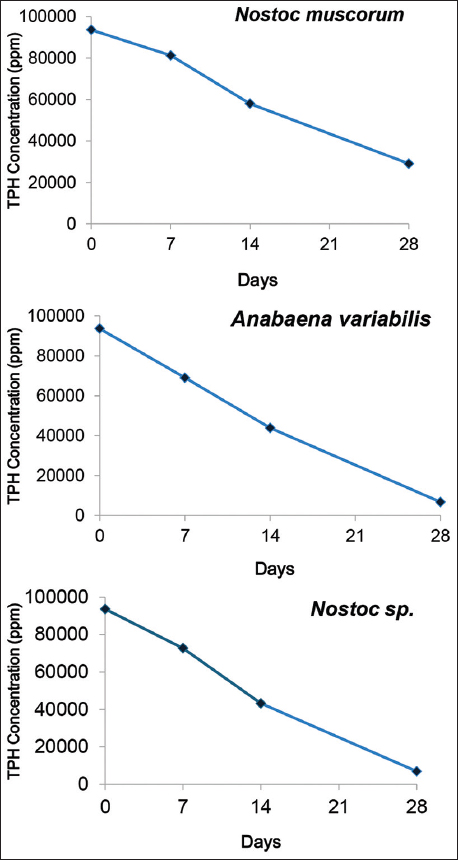 | Figure 8: Graphical representation of the biodegradation reaction kinetics by the three cyanobacterial cultures. [Click here to view] |
Further analysis of the kinetic model also revealed that A. variabilis and Nostoc spp. followed a faster first-order biodegradation process compared to N. muscorum, making them more efficient candidates for sludge bioremediation.
3.5. Enzyme Assays
The biodegradation ability of the cyanobacterium N. muscorum, A. variabilis, and Nostoc spp. was further experimented with the activity of some of the important enzymes as an indication of a functional biochemical and metabolic ability of microorganism that has managed to possible hydrocarbon utilization [Figure 9].
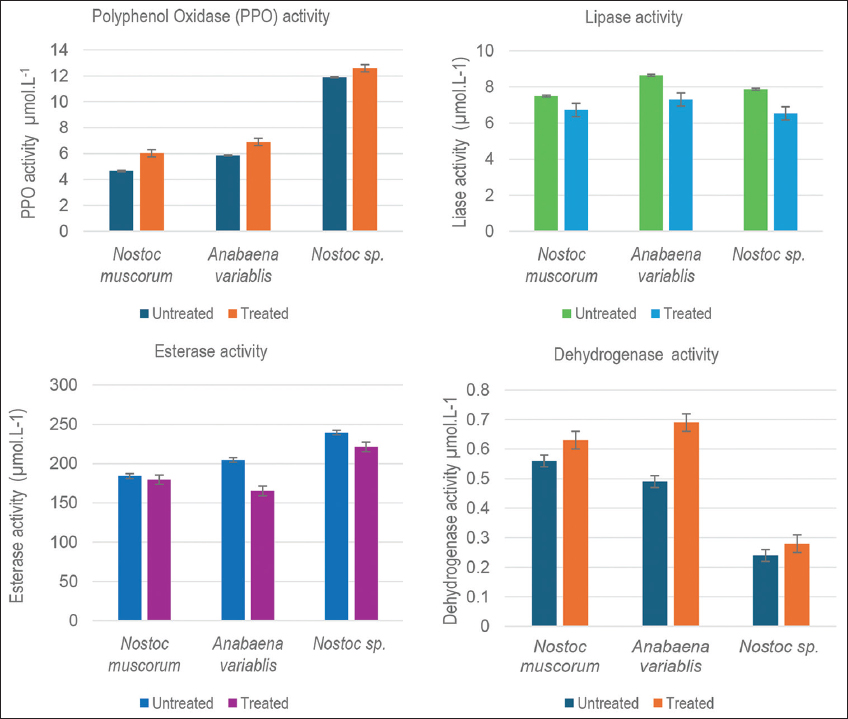 | Figure 9: The enzyme activities of the three sludge-treated cultures on the 28th day of incubation. The obtained values in a sample size n=6, in duplicates, are represented with their respective ± standard deviation values. [Click here to view] |
3.5.1. PPO
Oxidation of aromatic compounds in nature is an enzyme-dependent process, performed by a group of copper enzymes, PPO [25] with two major varieties: tyrosinases (EC 1.14.18.1) and laccases (EC 1.10.3.2) [26]. The activity of PPO enzyme in the present study exhibited accelerated growth upon treatment with sludge, confirming the better hydrocarbon utilization. On the 28th incubation day, an increase of 22.71%, 15.07%, and 5.48% was observed in the PPO activity by N. muscorum, A. variabilis, and Nostoc spp., respectively. Thus, it corroborates the fact that the ability of cyanobacteria to tolerate as well as remove phenol from different systems within days [27].
3.5.2. Catalase
The confrontation ability of filamentous cyanobacteria Nostoc punctiforme ATCC 29133 (Nostoc 29133) and Anabaena spp. PCC 7120 (Anabaena 7120) toward reactive oxygen species (ROS), by virtue of intrinsic constitutive catalase activity [28]. Catalase was identified as a biochemical marker of oxidative stress against hydrogen peroxide with a reported high activity in the marine cyanobacterium Synechococcus aeruginosus [29]. The catalase activity in the present study was recorded by either the presence or absence of effervescence, thus taken to be a qualitative physical parameter to mark the functionality of the enzyme in the cultures. This was due to the lack of a scalable amount of effervescence evolved in the reaction assay. On day 28th, the cultures, both treated and positive controls of the three cyanobacterial strains, exhibited a functional catalase enzyme metabolism, thus adding up to the inference of these cyanobacteria being capable of withstanding an oxidative stress that the tank bottom sludge could possibly pose.
3.5.3. Lipase activity
The screening of strains with a nature of degrading oil is determined by an important reference of lipase activity. Its role has been found to be indispensable for biodegradation analysis of oily wastes such as cooking oil [30,31]. The presence of ester bond-cleaving factor (or factors) in cultures composed of Acinetobacter lwoffii and Pseudomonas chlororaphis PA23 strains, supplied with substrates of p-nitrophenyl-fatty acid and various subunit compositions of PHA polymers [32]. In the present study, a positive, although decreased, activity of lipase was encountered by the three cyanobacterial cultures treated with tank bottom sludge waste. By the completion of the incubation period, the lipase activity by sludge-treated N. muscorum, A. variabilis, and Nostoc spp., decreased was by 10.26, 15.49, and 17.00 percent, respectively, as compared to their positive control cultures. It could therefore be inferred that an active lipase enzyme system present in these cultures could be facilitating the degradation of the sludge added to the cultures and that its activity decreases with the decrease in the concentration of sludge due to the degradation.
3.5.4. Esterase activity
An esterase activity depicts constant increase during the process of degradation of the dimethyl phthalate (DMP) [33], a kind of phthalic acid esters that are classified as a refractory organic plasticizer compound [34-36]. Although the esterase enzyme activity like that of PPO and lipase, in comparison to the dehydrogenase enzymes, was observed to accelerate greatly, the recorded activity of the treated cultures was slightly lesser than that of the positive control on completing the incubation. The variation in the activities could be due to the amount of specific enzymes, esterase being produced in the maximum amount, required to be produced by the cells in accustoming to the sludge samples it has been exposed to. The decrease observed was 2.52%, 19.31%, and 7.55% by N. muscorum, A. variabilis, and Nostoc spp., respectively. A similar observation was reported on microalgal cells of Pseudokirchneriella subcapitata, with an optimal intracellular esterase activity, met a 20 times higher toxic effect on exposure to oil sands process-affected water than to oil sands exposed to water, and a simultaneous stimulating effect on its photosynthetic function parameters [37]. From this output, it can be inferred that although a decrease, a highly functional enzymatic metabolism is performed by the three cultures, thereby supporting the possibility of efficient biodegradation of tank bottom sludge.
3.5.5. Dehydrogenase enzyme
The dehydrogenase enzyme assay is a prominent index for assessing microbial activity. In this study, the activity was observed to augment in all three treated cyanobacterial strains in comparison to the positive controls, by the 28th day of incubation. A. variabilis turned to have the highest activity of 0.69 ± 0.03μmol/L with an increase of 29%, followed by N. muscorum with 0.63 ± 0.02 μmol/L with an increase of 15% and Nostoc spp. with 0.28 ± 0.00 μmol/L with an increase of 12% from its respective positive control. Thus, in concurrence with an increased dehydrogenase activity by bacterial strains for the biodegradation of weathered hydrocarbons in engine oil-treated soil, it can be concluded that the studied cyanobacterial strains in this study do have the potential to degrade the tank bottom sludge introduced in the culture [38].
3.6. GC-MS Analysis
Based on the holistic inference, there exists no second thought that the biodegradation potential of Nostoc spp. is highly efficient in response to sludge hydrocarbon removal in comparison to the other cyanobacterium N. muscorum and A. variabilis. Thus, the processed sample from the untreated and the treated oil sludge by the culture of Nostoc spp. was investigated further through GC-MS. The samples were prepared with the same protocol depicted for GC-FID analysis and were sent to Zibsar Biotech, Guwahati, Assam, for GC-MS analysis. The analysis suggests that the biodegradation efficiency of various organic compounds, including hydrocarbons, sulfurous compounds, and polycyclic aromatic hydrocarbons (PAHs), described as before and after treatment in Table 2, is up scalable.
Table 2: A cluster of hydrocarbons and accessory compounds and their biodegradation indices detected through gas chromatography-mass spectrometry of the untreated and the treated oil sludge samples on the incubation for 28th days.
| Class | Compound type | ∑Compounds untreated (mg/mL) | ∑Compounds treated (mg/mL) | Biodegradation index (%) |
|---|---|---|---|---|
| Saturated | Aliphatic hydrocarbon | 24 | 10 | 58.33 |
| Cyclic hydrocarbon | 2 | 0 | 100.00 | |
| Unsaturated | Cyclic hydrocarbon | 6 | 3 | 50.00 |
| Amide | 1 | 0 | 100.00 | |
| Other functional groups | Ester | 5 | 0 | 100.00 |
| Anhydride | 1 | 0 | 100.00 | |
| Alcohol | 2 | 0 | 100.00 | |
| Terpenoid | 1 | 0 | 100.00 | |
| Acidic vitamin | 1 | 0 | 100.00 | |
| Sulfurous compounds | - | 15.48 | 1.48 | 90.43 |
| Polycyclic aromatic hydrocarbons | Naphthalene | 2 | 0 | 100.00 |
| Phenanthrene | 5 | 0 | 100.00 | |
| Pyrene | 1 | 0 | 100.00 |
The biodegradation efficiency of different classes of compounds was assessed by comparing their concentrations in untreated and treated samples with aliphatic hydrocarbons exhibited moderate degradation (58.33%), while cyclic hydrocarbons were completely degraded (100%). The difference suggests that straight-chain alkanes may require more time or optimized conditions for complete breakdown. Cyclic hydrocarbons showed partial degradation (50%), indicating possible resistance to cyanobacterial breakdown and esters, anhydrides, alcohols, terpenoids, and acidic vitamins were fully degraded (100%), demonstrating strong microbial enzymatic activity. Furthermore, the sulfurous compounds exhibited high degradation efficiency (90.43%), suggesting the presence of effective sulfur-metabolizing microbes and polycyclic aromatic hydrocarbons (PAHs) demonstrated complete degradation of naphthalene. Thus, from the obtained analysis, the culture’s ability is determined in regard to the objective of biodegradation of the sludge on incubation in the suitable culture medium, implying its reliability in being replicated on a larger scale.
4. DISCUSSION
Cyanobacteria by virtue of their stress management ability were reported for enhanced carotenogenic properties, facilitating the storage of highly cited carotenoids in the cells [39]. In addition, the phycobiliproteins were specifically reported for their exclusive occurrence in these blue-green algae [40]. The photosynthesis-aiding pigments, chlorophyll, present in the cyanobacterial cells are routinely employed for the growth monitoring and assessment of the cultures. Thus, in addition to the enzyme assays and GC-FID, this pigment profile was regularly monitored for 28 days to make a holistic approach for assessing the biodegradation potential of these cultures. In the course of incubation, the three cultures exhibited a specific pattern of initial decrease, followed by a stable increase in the Chl a and Chl b content, with Nostoc spp. with the best chlorophyll profile. In terms of the carotenoids, the three strains maintained a coinciding pigment profile, whereas the treated N. muscorum revealed a higher PC content than its positive control culture unlike Nostoc spp. and A. variabilis cultures with a comparatively lower yet decent phycobilisomes protein content than their respective positive controls. Although carotenoids and phycobilins are known to confer stress protection, their decline post-sludge exposure may signal their consumption during ROS detoxification or repression due to photodamage of these light-harvesting pigments under sludge-darkened or oil-coated reducing photosynthetic pigment pools due to light shading and resource reallocation to conserve energy or redirect metabolic flux toward stress-mitigating enzymatic pathways (e.g., catalase, dehydrogenases) during hydrocarbon bioremediation.
Cyanobacteria being one of the oldest lineages on the biosphere of the earth with having undergone all types of natural and anthropogenic stresses for billions of years have developed an advanced sensory mechanism and highly systematic metabolism [41]. The cellular organizations of these unicellular or multicellular, filamentous microbes enable them to combat the adverse living conditions in the terrestrial or marine environment. This study aimed to enforce the adaptive nature of these blue-green algae to quantify the transformation and degradation of the hydrocarbon composition of tank bottom oil sludge by monitoring their growth and enzymatic action on exposure to this highly toxic waste generated in tons across the globe by oil refineries.
As mentioned earlier, the sludge is composed of both aliphatic and aromatic hydrocarbons, [41] provided an evidence of an “enzymatic apparatus” in cyanobacterial cells suitable for biosynthesis of precursor compounds of aromatic ring-containing substances, thereby suggesting an ability to transform aromatic compounds such as secondary metabolite with the aid of this enzyme system, investigated through GC-FID and GC-MS. Thus, studying these enzyme systems becomes crucial as it serves as an index for quantifying the biotransformation and biodegradation of the tank bottom sludge exposed to the cultures, adding to the innovation aspect of this investigation by having the A. variabilis and N. muscorum along the Nostoc spp. to be reported for the bioremediation of tank sludge from oil refinery sites in particular. It remains an uncommon approach to identify the enzymatic mechanistic in detail because esterase and lipase enzymes are well-characterized in bacteria and fungi, and investigation in cyanobacterial cultures is commonly regarding their biomass or physiological phenomena, lacking intrinsic data on their enzymatic mechanism. The activity of esterase and lipase uncovers the specific catalytic action conducted by the cyanobacteria to remediate the long-chain hydrocarbons, revealing the enzyme systems that are upregulated, thus providing the mechanistic evidence for metabolic capacities, establishing them as biomarkers [42]. Selecting the optimized cells having superiority in the desired cellular functions by determining the enzymatic activity trends could boost the remediation. Cyanobacteria lack a conventional β-oxidation pathway [43] that is common in other microbes. Thus, the identification and quantification of such similar enzymes shall be aiding in illustrating the unique hydrocarbon remediation mechanism displayed by these cultures.
Intracellular enzymes such as PPO are inducible in nature and thus various species of microalgae have been investigated over the years for an induced degradation of phenolic compounds [33]. In fact, the first marine organism to extract PPO from was cyanobacterium Phormidium valderianum [26]. Tyrosinase and laccase are the two major varieties of PPO enzyme [26]. It was unveiled that laccase extracted from Chlamydomonas moewusii was the key enzyme responsible. Thus, this finding could be correlated with the present study, indicating a 22.71%, increase of polyphenol activity by the culture N. muscorum on the completion of the incubation period. The observed qualitative and quantitative upsurge in PPO activity upon sludge exposure a strong correlation can be inferred between PAH breakdown efficiency and PPO-mediated aromatic hydroxylation mimicking laccase/tyrosinase function. The presence of a tyrosinase-like activity capable of catalyzing ring hydroxylation – a preliminary step in PAH degradation is suggested by the upregulated PPO activity.
About all anthropogenic (herbicides, heavy metals, etc.) and natural (desiccation, high-intensity light, salinity, etc.) strains that cyanobacteria have encountered throughout the period of evolution have culminated in oxidative stress in many cases [44]. Numerous enzymes, of which peroxidases and catalases, are the two main families and detoxify the H2O2 produced within cells. It is interesting to note that catalases and peroxiredoxins are widely represented in cyanobacteria [45], like glutathione peroxidases and ascorbate peroxidases, which are important in breaking down H2O2 in mammals and plants, respectively, as they are broadly present [46]. In agreement with these reports, the treated along with the positive control cultures in this study exhibited a functional catalase enzyme system on the 28th day of incubation, indicating the combating ability of the cultures toward the stress posed by the tank bottom oil sludge.
Lipases are categorized as ester hydrolases capable of cleaving ester bonds along with having a conserved lipase box and the catalytic triad of histidine, serine, and aspartate residues [32]. Microorganisms that produce lipase have a great deal of scientific and practical significance since they may both directly break down waste oil and encourage the breakdown of oily waste. This suggests that the role of lipase is highly significant in the biodegradation of oily wastes like those from kitchens and that it is necessary for the strains of bacteria that can break down waste oil [47]. The function of the lipase in this study for the breakdown of the oily sludge was assessed and on the 28th day, an observed decreased activity was inferred to be proportional with the decrease in the concentration of the sludge broken down during the course of incubation.
A specific group of enzymes, esterase (EC 3.1.1.2), with an ester hydrolysing nature, are essential for a primary attack on xenobiotics such as phthalates and reports indicated three freshwater unicellular cyanobacteria Cyanothece spp. PCC7822, Synechococcus spp. PCC7942, and Synechocystis spp. PCC6803 performing enhanced esterase activity during biodegradation of DMP a precursor of a more toxic metabolic intermediate mono-methyl phthalate causing endocrine disruption and interference in the development and reproductive system of animals as well as humans [33]. These reports justify the significance to study the esterase in the sludge-treated cultures. The observation from the performed assays indicated a reduction in the activity with N. muscorum, showing the lowest decrease in comparison to their respective controls. Although a reduction was encountered, it is noteworthy that in comparison to the dehydrogenase, PPO, and lipase enzymes, esterase was contained in a high amount by all the three cultures. In concurrence with the findings [37], it could be inferred that toxicity constituted by the oily sludge was responsible for the decreased activity and that the cultures are composed of a functional esterase enzyme system contributing to the biodegradation of the sludge.
A unique intracellular enzyme in most soil microbes, dehydrogenase, is often used as the key to measure microbial activity as its functionality serves as the estimate of the complete oxidation potential of these microbes [48-50]. This defines the necessity of the dehydrogenase assay in this study, where a rise of 29%, 15%, and 12% was observed by A. variabilis, N. muscorum, and Nostoc spp., respectively, treated with tank bottom oil sludge. The dehydrogenase activity is associated with oxidative transformations of aromatic hydrocarbons by facilitating the hydroxylation and activation before ring cleavage by the PPOs. Therefore, with the support of these values, inference could be made that the enzyme facilitated the degradation of the sludge positively.
Collectively, the observations continue to support the aim of this study with substantial findings. Significant reductions in the TPH content with the evidence of reduced peak area of the hydrocarbon spectrum of the treated oil sludge on exposure to the three filamentous cyanobacterial cultures, Nostoc spp. being the highest efficient from its additional GC-MS inferences suggest that these strains are immensely capable of remediating bulk tank bottom oil sludge for its safe disposal into the environment without giving rise to secondary pollutants or toxins or threat to surrounding living organisms or deteriorating the soil or water quality. The TPH reduction efficiency for the cyanobacterial cultures displayed as high as >99% by A. variabilis and Nostoc spp., having N. muscorum lagging slightly at ~94%. The reasons like ineffective enzyme isoforms or affinities for the spectrum of the hydrocarbons in the tank sludge, or inadequate expression of the co-factors and transporters, or physiological attributes such as cell wall permeability, formation of microcolonies, or intracellular storage limiting efficient uptake, processing, or excretion of degradation products could be causing the lag in the bioremediation of the sludge by the N. muscorum culture. The total degradation of cyclic hydrocarbons (100%) compared to the limited degradation of aliphatic hydrocarbons (58.3%) specifies the substrate-specific enzymatic affinity in the cyanobacterial cultures. This significant disparity in degradation due to the substrate-specific limitations in the cyanobacterial enzymatic repertoire aids in oxidoreductive cleavage of ring structures over linear alkanes, which typically demand monooxygenase systems absent in cyanobacteria. This is because these are more susceptible to enzymatic attack by PPOs and dehydrogenases, which were shown to be significantly upregulated in this study, especially in A. variabilis and Nostoc spp. The limited beta-oxidation pathway in cyanobacteria [43] affects the efficient breakdown of long-chain aliphatic hydrocarbons. Hence, the partial degradation of saturated aliphatics – despite otherwise high TPH removal rates (>99%) – can be attributed to this biochemical bottleneck in enzymatic compatibility with long-chain alkanes, or suitability of the linear hydrocarbons for their suitability as nutritional sources, intensifying the cellular growth [51-53]. The high degradation rates by Nostoc spp. and A. variabilis highlight the potential that these cyanobacterial cultures surpass microbial consortia. A 85–92% TPH reduction was asserted by Hamouda et al. [1] using an algae consortium – indicating that these particular cyanobacteria strains perform better or at least comparably well individually than typical multi-algal systems documented in literature.
It is a holistic study designs that lead to the unveiling of the strengths and the lag of the catalytic machinery through enzymatic, cellular physiological adaptation, and regulating parameters. Thus, it is a substantial step forward in depicting the potential of these cyanobacterial cultures in the objective of bioremediation that is up scalable for a practical appliance.
5. CONCLUSIONS
The present study underscores the potential of bioremediation as a sustainable approach in combating refinery waste using cyanobacteria as a cost-effective methodology. In addition, the present study emphasizes on the optimizing environmental conditions to enhance the degradation rates and exploring metagenomics and enzyme profiling for deeper insights into biodegradation pathways.
6. FUNDING SUPPORT
The authors acknowledge the support extended by the Numaligarh Refinery Limited (NRL), Jorhat, Assam, for sponsoring the project to Dr Samrat Adhikari and Mr Sumit Deb. The authors also expressed their gratitude to the Advanced Level Biotechnology Research Hub funded by DBT. Govt. of India, at St. Edmund’s College, Shillong, for allowing access to its facility.
7. AUTHOR’S CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All data generated and analyzed are included within this research article
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
12. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Hamouda RA, Alhumairi AM, Saddiq AA. Simultaneous bioremediation of petroleum hydrocarbons and production of biofuels by the micro-green alga, Cyanobacteria, and its consortium. Heliyon. 2023;9:16656.[CrossRef]
2. Brock TD, Carr NG, Whitton BA. The Biology of Blue-Green Algae. Oxford:Blackwell Scientific Publications;1973.
3. Mona S, Kumar V, Deepak B, Kaushik A. Cyanobacteria:The eco-friendly tool for the treatment of industrial wastewaters. In:Bharangava RK, Saxena G, editors. Bioremediation of Industrial Waste for Environmental Safety. Singapore:Springer;2020.[CrossRef]
4. Bano S, Burhan ZUN, Nadir M, Ahmed A, Rasool SG, Siddiqui JJA, et al. Removal efficiency of marine filamentous cyanobacteria for pyrethroids and their effects on the biochemical parameters and growth. Algal Res. 2021;60:102546.[CrossRef]
5. Zyszka-Haberecht B, Niemczyk E, Lipok J. Metabolic relation of cyanobacteria to aromatic compounds. Appl Microbiol Biotechnol. 2019;103(3):1167-78.[CrossRef]
6. Dudeja C, Masroor S, Mishra V, Kumar K, Sansar S, Yadav P, et al. Cyanobacteria-based bioremediation of environmental contaminants:Advances and computational insights. Discov Agric. 2024;3:42.[CrossRef]
7. Bhattacharyya JK, Shekdar AV. Treatment and disposal of refinery sludges:Indian scenario. Waste Manag Res. 2003;21(3):249-61.[CrossRef]
8. Dell'Anno A, Rastelli E, Sansone C, Brunet C, Ianora A, Dell'Anno A. Bacteria, fungi and microalgae for the bioremediation of marine sediments contaminated by petroleum hydrocarbons in the omics era. Microorganisms. 2021;9:1695.[CrossRef]
9. Stepanova AY, Gladkov EA, Osipova ES, Gladkova OV, Tereshonol DV. Bioremediation of soil from petroleum contamination. Processes. 2022;10:1224.[CrossRef]
10. Patel A, Tiwari S, Prasad SM. Arsenate and arsenite-induced inhibition and recovery in two diazotrophic cyanobacteria Nostoc muscorum and Anabaena sp.:Study on time-dependent toxicity regulation. Environ Sci Pollut Res. 2021;28:51088-51104.[CrossRef]
11. Hasan AMA, Kamal RS, Farag RK, Abdel-Raouf ME. Petroleum sludge formation and its treatment methodologies:A review. Environ Sci Pollut Res. 2024;31:8369-86.[CrossRef]
12. El-Sheekh MM, Hamouda RA. Biodegradation of crude oil by some cyanobacteria under heterotrophic conditions. Desalin Water Treat. 2024;52(7-9):1448-54.[CrossRef]
13. Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. Microbiology. 1979;111(1):1-61.[CrossRef]
14. Jungai N, Adhikari S. Genetic diversity of free-living filamentous cyanobacteria isolated from a variety of coal mining areas of Jaintia Hills District, Meghalaya, India. Int J Res Stud Biosci. 2015;3(12):26-34.
15. Negi Y, Sharma S, Sutradhar N, Adhikari S. A study on the differentiation of filamentous cyanobacterial isolates using DNA fingerprinting approach. Indian J Biotechnol. 2019;18(4):337-45.
16. Senger H. Characterization of a synchronous culture of Scenedesmus obliquus, its potential photosynthetic capacity and its photosynthetic quotient during the life cycle. Planta. 1970;90(3):243-66.[CrossRef]
17. Li Y, Huang X, Luo L, Shang C. Optimization of extraction conditions of carotenoids from Dunaliella parva by response surface methodology. Molecules. 2022;27(4):1444.[CrossRef]
18. Bennett A, Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973;58(2):419-35.[CrossRef]
19. Suganthi SH, Murshid S, Sriram S, Ramani K. Enhanced biodegradation of hydrocarbons in petroleum tank bottom oil sludge and characterization of biocatalysts and biosurfactants. J Environ Manage. 2018;220:87-95.[CrossRef]
20. Mayer AM, Harel E. Polyphenol oxidase in plants. Phytobiodeterioration Biodegradation. 1979;33:129-43.
21. Iwase T, Tajima A, Sugimoto S, Okuda K, Hironaka I, Kamata Y, et al. A simple assay for measuring catalase activity:A visual approach. Sci Rep.2013;3:3081.[CrossRef]
22. Válek T, Pohanka M. The determination of lipase activity by measuring pH using ion-sensitive field-effect transistor. Int J Electrochem Sci. 2021;16:210760.[CrossRef]
23. Peng Y, Fu S, Liu H, Lucia LA. Accurately determining esterase activity via the isosbestic point of p-nitrophenol. BioRes. 2016;11(4):10099-111.[CrossRef]
24. Nachlas MM, Margulies SI, Goldberg JD, Seligman AM. The determination of lactic dehydrogenase with a tetrazolium salt, Anal Biochem. 1960;1(4-5):317-26.[CrossRef]
25. Solano F, Sanchez-Amat A. Studies on the phylogenetic relationships of melanogenic marine bacteria:Proposal of Marinomonas mediterranea sp. . Int J Syst Bacteriol. 1999;49:1241-6.[CrossRef]
26. Patel AB, Shaikh S, Jain KR, Desai C, Madamwar D. Polycyclic aromatic hydrocarbons:Sources, toxicity, and remediation approaches. Front Microbiol. 2020;11:562813.[CrossRef]
27. Shashirekha S, Uma L, Subramanian G. Phenol degradation by the marine cyanobacterium Phormidium valderianum BDU 30501. J Ind Microbiol Biotechnol. 1977;19:130-3.[CrossRef]
28. Samanta L, Stensjo K, Lindblad P, Bhattacharya J. Differential catalase activity and tolerance to hydrogen peroxide in the filamentous cyanobacteria Nostoc punctiforme ATCC 29133 and Anabaena sp. PCC 7120. Arch Microbiol. 2022;204(2):121.[CrossRef]
29. Hussain JM, Muruganantham P, Abdul Kareem KA. Hydrogen peroxide stress induced in the marine cyanobacterium Synechococcus aeruginosus and Phormidium valdarianum. Appl Biochem Biotechnol. 2023;196:522-36. [CrossRef]
30. Liu D, Ma X, Huang J, Shu Z, Chu X, Li Y, et al. Study on personalized microbial formulation during high-temperature aerobic fermentation of different types of food wastes. Sci Total Environ. 2022;814:152561.[CrossRef]
31. Ke X, Sun JC, Liu C, Ying JM, Zou SP, Xue YP, et al. Fed-in-situ biological reduction treatment of food waste via high-temperature-resistant oil degrading microbial consortium. Bioresour Technol. 2021;340:125635.[CrossRef]
32. Sharma PK, Mohanan N, Sidhu R, Levin DB. Colonization and degradation of polyhydroxyalkanoates by lipase-producing bacteria. Can J Microbiol. 2019;65(6):461-75.[CrossRef]
33. Zhang X, Liu L, Zhang S, Pan Y, Li J, Pan H, et al. Biodegradation of dimethyl phthalate by freshwater unicellular Cyanobacteria. Biomed Res Int. 2016;2016:5178697.[CrossRef]
34. Luo ZH, Wua YR, Chowa RKK, Luo JJ, Gu JD, Vrijmoed LLP. Purification and characterization of an intracellular esterase from a Fusarium species capable of degrading dimethyl terephthalate.Process Biochem.2012;47(5):687-93.
35. Wang J, Luo Y, Teng Y, Ma W, Christie P, Li Z. Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environ Pollut. 2013;180:265-73.[CrossRef]
36. Wang WL, Wu QY, Wang C, He T, Hu HY, Health risk assessment of phthalate esters (PAEs) in drinking water sources of China. Environ Sci Pollut Res. 2015;22(5):3620-30.[CrossRef]
37. Debenest T, Turcotte P, Gagne F, Gagnon C, Blaise C. Ecotoxicological impacts of effluents generated by oil sands bitumen extraction and oil sands lixiviation on Pseudokirchneriella subcapitata. Aquat Toxicol. 2022;112-3:83-91.[CrossRef]
38. Ramadass K, Megharaj M, Venkateswarlu K, Naidu R. Bioavailability of weathered hydrocarbons in engine oil-contaminated soil:Impact of bioaugmentation mediated by Pseudomonas spp. bioremediation. Sci Total Environ. 2018;636:968-74.[CrossRef]
39. Ribeiro JES, Martini M, Altomonte I, Salari F, Nardoni S, Sorce C, et al. Production of Chlorella protothecoides biomass, chlorophyll and carotenoids using the dairy industry by-product scotta as a substrate. Biocatal Agric Biotechnol. 2017;11:207-13.[CrossRef]
40. Deviram G, Mathimani T, Anto S, Shan Ahamed T, Arul Ananth D, Pugazhendhi A. Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J Clean Prod. 2020;253:119770.[CrossRef]
41. Zyszka-Haberecht B, Niemczyk E, Lipok J. Metabolic relation of Cyanobacteria to aromatic compounds. Appl Microbiol Biotechnol. 2019;103(3):1167-78.[CrossRef]
42. Mishra A, Gupta J, Kumari T, Pal R, Thakur IS. Unravelling the attributes of novel cyanobacteria Jacksonvillea sp. ISTCYN1 by draft genome sequencing. Bioresour Technol. 2021;337:125473.[CrossRef]
43. Berlepsch S, Kunz HH, Brodesser S, Fink P, Marin K, Flügge UI, et al. The acyl-acyl carrier protein synthetase from Synechocystis sp. PCC 6803 mediates fatty acid import. Plant Physiol. 2012:159(2):606-17.[CrossRef]
44. Dadheech N. Desiccation tolerance in Cyanobacteria. Afr J Microbiol Res. 2010;4(15):1584-93.
45. Zamocky M, Furtmüller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxid Redox Signal. 2008;10(9):1527-48.[CrossRef]
46. Tel-Or E, Huflejt M, Packer L. The role of glutathione and ascorbate in hydroperoxide removal in Cyanobacteria. Biochem Biophys Res Commun. 1985;132(2):533-9.[CrossRef]
47. Jiang S, Fan Q, Zhang Z, Deng Y, Wang L, Dai Q, et al. Biodegradation of oil by a newly isolated strain Acinetobacter junii WCO-9 and its comparative pan-genome analysis. Microorganisms, 2023;11(2):407.[CrossRef]
48. Alrumman SA, Standing DB, Paton GI. Effects of hydrocarbon contamination on soil microbial community and enzyme activity. J King Saud Univ Sci. 2015;27:31-41.[CrossRef]
49. Ramadass K, Megharaj M, Venkateswarlu K, Naidu R. Ecological implications of motor oil pollution:Earthworm survival and soil health. Soil Biol Biochem. 2015;85:72-81.[CrossRef]
50. Richardson EL, King CK, Powell SM. The use of microbial gene abundance in the development of fuel remediation guidelines in polar soils. Integr Environ Assess Manag. 2015;11(2):235-41.[CrossRef]
51. Wyszkowska J, Kucharski J. Correlation between the number of cultivable microorganisms and soil contamination with diesel oil. Pol J Environ Stud. 2005;14:347-56.
52. Kucharski J, Jastrzebska E. Effects of heating oil on the count of microorganisms and physico-chemical properties of soil. Pol J Environ Stud. 2005;14(2):195-204.
53. Kadri T, Rouissi T, Magdouli S, Brar SK, Hegde K, Khiari Z, et al. Production and characterization of novel hydrocarbon degrading enzymes from Alcanivorax borkumensis. Int J Biol Macromol. 2018;112:230-40.[CrossRef]