1. INTRODUCTION
Nowadays, the ready-to-drink products were very popular in the world [1]. Consuming too much ready-to-drink product, however, could increase obesity and chronic diseases and become a serious issue in many countries [2]. Consumers are finding healthier food and beverages [3]. Natural fruit juice drink, therefore, is getting a lot of attentions in beverage market and becomes a new trend among young consumers all over the world.
Fruits are vital sources of dietary supplement research as therapeutic agents. The demand for functional and health support products has grown considerably in recent years. Fruits are rich in antioxidant properties and play important roles for human health [4]. Fruits can reduce cholesterol and hormonal metabolism, improve immune system, affect on modulation of steroid hormone concentration and detoxifying enzymes, reducing blood pressure, antibacterial stimulation, antioxidant activity, anti-carcinogenic properties, and cardiovascular diseases retardation [5].
Flacourtia jangomas is a rainforest fruit and widespread in Asia countries such as China, India, Bangladesh, Myanmar, Malaysia, Indonesia, Taiwan, Thailand, and Vietnam [6]. In Vietnam, this plant generally only grows in mountainous or middle-earth regions, bearing fruit once a year in August and September from the solar calendar. The Hong Quan fruit has sweet and sour taste. It contains carbohydrates, proteins, fats, ascorbic acids, tartaric acid, beta-carotene, B3 vitamin, and minerals such as Ca, K, P, Fe, and Mg. Moreover, this fruit also contains alkaloids, phenolics, flavonoids, and tannins that have antioxidant, anti-inflammatory, analgesic, antibacterial, antidiarrheal, antiviral, and inhibiting amylase enzymatic activities; thus it contributes to the treatment of diabetes [7-12]. Hong Quan fruit has been used as fresh fruit by local residents. In Tinh Bien which is mountainous district of An Giang province, F. janomas plant is cultivated extensively. It can be considered as an abundant source for processing high nutritious and organic valued beverages. This work aims to increase diversity of beverage market and creates new locally specific drinks.
Pasteurization was known as a thermal process that is often applied in food industry to guarantee food safety. Temperature and heating time of pasteurization are important factors in processing. High temperature damage heat-sensitive nutrients, biochemical and biological reactions also can occur and reduce product quality [2]. Therefore, this research was conducted to determine the most optimal pasteurized parameters for the Hong Quan beverage to retain its bioactive ingredients.
2. MATERIALS AND METHODS
2.1. Materials
Hong Quan fruits were collected in Tinh Bien district, An Giang province, Vietnam (0° 36’ 0” North, 104° 57’ 0” East) on April 2022. The fruits were delivered to the laboratory within 3 h. Then good quality fruits were washed, drained, divided into plastic bags (Polyethylene – PE). Each bag contained 1 kg, then the bags were stored in the freezer (Inverter Sanaky 761 Liter VH 8699HY3) at temperature ≤ −18oC.
When the experiment was carried out, the fruits were defrosted by soaking in tap water for 5 min. Hong Quan fruits were finely ground (model: Philips HR2223/00) for 5 min with the ratio of water/fruit was 8/1 (v/w) and roasted peanuts were added at 14% (w/w) compared to the fruits. Fruit puree was filtered through a sieve (θ 0.6 mm) to remove residue, then treated with pectinase enzyme at concentration of 0.1% (v/v) at 60oC for 45 min. The puree then was mixed with CMC (0.3%) and xanthan gum (0.25%), adjust Brix to 14o and pH 3.8. The mixture was heated at 80oC for 10 min to dissolve the substances, filling in glass bottles (250 mL) with lids. Hong Quan juice bottles were pasteurized at design temperature and time [Table 1]. The product then cooled down and analyzed the criteria.
Table 1: Coded and real values of pasteurization temperature and time.
| Number runs | Factors | |
|---|---|---|
| Temperature (°C) | Time (min) | |
| 1 | 90(0) | 18(-α) |
| 2 | 90(0) | 25(0) |
| 3 | 83(-α) | 25(0) |
| 4 | 95(+1) | 20(-1) |
| 5 | 95(+1) | 30(+1) |
| 6 | 90(0) | 25(0) |
| 7 | 85(-1) | 20(-1) |
| 8 | 90(0) | 32(+α) |
| 9 | 85(-1) | 30(+1) |
| 10 | 97(+α) | 25(0) |
| 11 | 90(0) | 25(0) |
2.2. Experimental Design
The effects of the pasteurization temperature (83, 85, 90, 95, and 97oC) and treating time (18, 20, 25, 30, and 32 min) on bioactive compounds (phenolic, flavonoid, tannin, and Vitamin C), and antioxidant activity (2,2-Diphenyl-1-picrylhydrazyl [DPPH], and ferric reducing antioxidant power [FRAP] assay) of product, a full factorial design (22+ star) was applied with three replicates at the center point with three blocks. These points fit with the surface plot for the responses and could estimate the pure errors of the multiple regression models [13], there were 11 samples were prepared [Table 1]. The ranges of temperature and pasteurization time in this work were based on preliminary studies.
Experimental designs and data were statistically analyzed using Statgraphics plus XVI. A quadratic equation was used to fit the results (Equation 1):
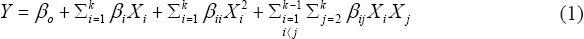 |
Where Y is the predicted response parameter, βo is a constant, βi, βii, and βij are the regression coefficients and Xi and Xj are the levels of the independent variables (pasteurization temperature and time).
2.3. Analysis Methods
2.3.1. Determination of phenolic content
The phenolics were determined by Folin–Ciocalteu method and the results were showed as milligrams of gallic acid equivalents per 100 g of product (mgGAE/100 g) based on the standard curve (y = 0.0082x + 0.0595, r2 = 0.9996) [14]. Each ethanol extracted sample (0.2 mL) was taken in a test tube and added 10% Folin–Ciocalteu reagent (1.5 mL). Then, all test tubes were kept in a dark place for 5 min. Finally, 5% Na2CO3 (1.5 mL) was added to solution and mixed well in a vortex. Again, all the test tubes were kept in the dark for 2 h. The absorbance was measured for all solution using UV-spectrophotometer at constant wavelength 750 nm.
2.3.2. Determination of flavonoid content
The aluminum chloride colorimetric method was exploited to determine flavonoids as quercetin equivalent per 100 g (mgQE/100 g) based on the standard equation y = 0.0054x + 0.0026, r2 = 0.9995 [15]. About 1 mL of the ethanol extracted sample/standard of different concentration solution was mixed with 3 mL ethanol, 0.2 mL of 10% aluminum chloride, 0.2 mL of 1 M sodium acetate, and 5.8 mL of distilled water. It remained at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm with spectrophotometer against blank.
2.3.3. Determination of tannin content
Tannin was determined by Folin-Denis method and the results were shown as milligrams of tannic acid equivalents per 100 g (mgTAE/100 g) based on standard curve (y = 0.0098x + 0.0478, r2 = 0.9996) [16]. Each ethanol extracted sample (0.5 mL) and distilled water (0.5 mL) were taken in a test tube. Finally, the samples were treated with 0.5 mL of freshly prepared Folin-Denis reagent and 20% of sodium carbonate (2 mL) was added, shaken well, warmed on boiling water-bath for 1 min and cooled to room temperature. Absorbance of the colored complex was measured at 700 nm.
2.3.4. Determination of vitamin C content
Vitamin C content was estimated according to the method described by Talreja [17], and result was presented as milligrams per 100 g of product. Take 0.1 g of sample in a test tube, add 2 mL of 2% MPA and leave for 1 h. Then centrifuge at 2500 rpm for 15 min. Take 1 mL of the clear solution from the top of the test tube and add 2 mL of 5% MPA, 5 mL of n-amyl alcohol, and 3.2 mL of 2–4-dichlorophenol indophenol reagent. Shake the sample well and measure the color at 546 nm. For the blank sample, 1 mL of distilled water, 2 mL of 5% MPA, 5 mL of n-amyl alcohol, and 3.2 mL of distilled water will be used. The ascorbic acid content in 1 mL will be calculated as follows: Y = 0.1103 – (0.14*OD). Where: Y is the concentration of ascorbic acid (mg/mL); OD is the absorbance of the sample at 546 nm.
2.3.5. Determination of FRAP assay
The iron reduction capacity was determined by FRAP assay [18]. The results were calculated against the FeSO4.7H2O standard curve (y = 0.5177x + 0.0855, r2 = 0.9981), and expressed as micromol ferric sulfate (μM FeSO4) per gram of product. The stock solutions included 300 mM acetate buffer (pH 3.6), 10 mM TPTZ (2, 4, 6-tripyridyl-s-triazine) solution in 40 mM HCl, and 20 mM FeCl3·6H2O solution. The fresh working solution was prepared by mixing 25 ml acetate buffer, 2.5 ml TPTZ, and 2.5 ml FeCl3·6H2O. The temperature of the solution was raised to 37°C before use. Test samples (150 μL) were allowed to react with 2850 μl of the FRAP solution for 30 min in the dark condition. Readings of the colored product (ferrous tripyridyltriazine complex) were taken at 593 nm.
2.3.6. Determination of DPPH assay
The free radical scavenging capacity was determined using DPPH as described by Aluko et al. [19]. One milliliter of 0.135 mM of DPPH in ethanol was mixed with 1 mL of test solution. The mixture was kept in a dark cupboard for 30 min. The absorbance of the resulting solution was measured spectrophotometrically at 517 nm and the scavenging ability of the extract was calculated as: DPPH (%) = ([Abs control – Abs sample]/Abs control) × 100. Where, Abs control is the absorbance of DPPH solution mixed in ethanol; Abs sample is the absorbance of extracts + DPPH solution.
2.4. Statistical Analysis
The optimum levels of the factors in pasteurization process of Hong Quan beverage were determinated with response surface methodology (RSM). The samples were pasteurized as experimental design to achieve high bioactive compounds and antioxidant activity using Statgraphics software. The data were statistically analyzed using analysis of variance (ANOVA) and means that were statistically different separated using least significant difference at P ≤ 0.05. The experimental data were established by quadratic model. A simultaneous optimization using the desirability function was performed to maximize the bioactive compounds content and antioxidant activity.
3. RESULTS AND DISCUSSIONS
3.1. Effect of Temperature and Pasteurization Time on the Bioactive Compounds of the Ready-to-drink Beverage from Hong Quan (F. jangomas) Fruit
Pasteurization that is an important process in beverage processing, affects not only microbial safety but also product quality, particularly bioactive compounds. To limit the reduction in product quality, it is necessary to find the optimum combination of both factors “temperature – time”. The effect of temperature and pasteurization time on the bioactive compounds is shown in Figure 1.
 | Figure 1: Response surface and contour plots for phenolic (a), flavonoid (b), tannin (c), vitamin C (d) at different temperatures, and pasteurization times. [Click here to view] |
Plots in Figure 1 showed that temperature and pasteurization time clearly affected the bioactive compounds content of the ready-to-drink beverage from Hong Quan fruit. The bioactive compounds such as phenolic, flavonoid, tannin, and Vitamin C tended to increase and reached to the highest level at 90oC, then slightly decreased at 95oC. Similar results were obtained with increasing the pasteurization time. These bioactive compounds were also an increase and reached a high value during 25 min, then slightly decreased at 30 min. The separated optimal values of phenolic, flavonoid, tannin, and Vitamin C were 28.98 mgGAE/100 g at 90.51oC and 24.12 min; 7.11 mgQE/100 g at 90.28oC and 26.48 min; 102.11 mgTAE/100 g at 90.45oC and 24.56 min, and 15.91 mg/100 g at 89.00°C and 23.26 min, respectively.
During the pasteurizing, by increasing the temperature, the bioactive compounds increase a first; however, if temperature keeps increasing, the amount of bioactive compounds then decreased. This may be due to phenolic properties of being sensitive to temperature and easily degraded by light and high temperatures [20]. The stability of phenolic and flavonoid depend on the temperature and pasteurization time and their molecular structure [21]. In addition, increased temperature increases the permeability and transferring of bioactive compounds from the fruit cell wall [22]. Moreover, as the pasteurization temperature increased, the flavonoid content increased due to the increased diffusion rate [20]. However, if the pasteurization keeps to increase up to 95oC, the amount of bioactive compound decreased, because phenolic compounds have been degraded by high temperatures. Besides, Laslo et al. [23] reported that there was a significant increase in the level of bioactive compounds with an extension of pasteurization time from 0 to 25 min at 80oC. Similar result was obtained in the study of Saini et al. [24], phenolic content increased from 15 to 45 min and decreased to 60 min.
3.2. Effects of Temperature and Pasteurization Time on the Antioxidant Activities of the Ready-to-drink Beverage from Hong Quan (F. jangomas) Fruit
There were many methods to measure the total antioxidant capacity in plant extracts and biological fluids [25]. In this study, the FRAP and radical scavenging (DPPH) were selected to evaluate the antioxidant activity of Hong Quan beverage. Because FRAP is a highly sensitive method to measure the total antioxidant capacity of pharmacological plant products [26]. In Addition, DPPH is a stable and popular in studies of natural antioxidants [27]. The effect of temperature and pasteurization time on the antioxidant activities is shown in Figure 2.
 | Figure 2: Response surface and contour plots for DPPH radical scavenging (a), Ferrous reducing antioxidant power (FRAP) (b) at different temperatures and pasteurization times. [Click here to view] |
Plots in Figure 2 showed that when the pasteurization temperature changed from 85oC to 95oC, FRAP of the ready-to-drink beverage made from Hong Quan fruit tended to increase and reached to the highest value at 90oC [Figure 2b], while DPPH reached the highest value at 95oC. In case of the pasteurization time, when it was extended from 20 to 30 min, FRAP and DPPH also tended to increase and reached the highest value which was at 25 min; after that, it was reduced as the time was increased to 30 min. The optimal DPPH and FRAP results were 85.88% at 91.19oC and 25.41 min; and 8.06 μM FeSO4/g at 90.19oC and 25.57 min, respectively.
Results of Farahmand et al. [28] and Benjamin et al. [29] reported that there was increase in antioxidant capacity of pomegranate juice was due to an increase in bioactive compounds such as phenolic, flavonoid, and anthocyanin. Figure 2 showed that when increasing the temperature and pasteurization time from 85oC to 90oC and 20–25 min, the content of bioactive compounds (phenolic, flavonoid, tannin, and Vitamin C) was positively correlated and causes the increases of antioxidant capacity of Hong Quan drink. In addition, the study by Zhang et al. [30] showed that the formation of the Maillard reaction during heat treatment also increased the antioxidant capacity. However, with the increases of temperature and pasteurization time, the antioxidant capacity of the product has been reduced due to bioactive compounds loss [21].
The regression coefficient of linear, second-order polynominal and in interaction effects, and regression models for bioactive compounds and antioxidant activity are described in Table 2.
Table 2: ANOVA analysis and regression coefficients of the linear, second-order polynomial and interaction effects and regression models for bioactive compounds and antioxidant activity.
| Coefficient | Estimate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic | P-value | Flavonoid | P-value | Tannin | P-value | Vitamin C | P-value | DPPH | P-value | FRAP | P-value | |
| Constant | −1607.88 | −361.61 | −2250.74 | −581.37 | −987.93 | −379.93 | ||||||
| X1 | 32.8161 | 0.0425 | 7.7347 | 0.0135 | 48.8546 | 0.0064 | 13.8183 | 0.0172 | 20.4933 | 0.0156 | 7.6727 | 0.0488 |
| X2 | 12.5789 | 0.0237 | 1.4769 | 0.0082 | 11.6676 | 0.0442 | −1.5179 | 0.0178 | 10.9686 | 0.0492 | 3.2844 | 0.0247 |
| X1X1 | −0.1701 | 0.0009 | −0.0417 | 0.0006 | −0.2615 | 0.0002 | −0.0825 | 0.0015 | −0.0996 | 0.0069 | −0.0398 | 0.0013 |
| X1X2 | −0.0842 | 0.0052 | −0.0078 | 0.0218 | −0.063 | 0.0057 | 0.0374 | 0.0099 | −0.0917 | 0.0114 | −0.0196 | 0.0074 |
| X2X2 | −0.1028 | 0.0025 | −0.0146 | 0.0045 | −0.1215 | 0.0011 | −0.0389 | 0.0065 | −0.0513 | 0.0253 | −0.0297 | 0.0023 |
| Lack-of-fit | 0.1645 | 0.0506 | 0.3375 | 0.8152 | 0.1046 | 0.8095 | ||||||
| R-squared (%) | 98.8171 | 97.1876 | 99.8246 | 99.6795 | 91.888 | 99.7371 | ||||||
| R-squared (%) (adjusted for d.f.) | 97.6342 | 94.3752 | 99.6493 | 99.359 | 83.776 | 99.4743 | ||||||
X1: Pasteurization temperature (oC); X2: Pasteurization time (min), R2adj: R-square adjusted determination coefficient
The previous researches [31,32] concluded that the goodness-of-fit of the regression model were fitted and its coefficient of determination R2 was over 0.8 and value of lack-of-fit that means non-significant at P > 0.05. The results of ANOVA analysis showed that the linear, quadratic and interaction factors of pasteurization temperature and time had effect on the content of phenolic, flavonoid, tannin, Vitamin C, and antioxidant activities (DPPH and FRAP assay) of obtained product with reliability 95%. The R values showed the coefficient of predicted models in the response was R2 > 0.91 and lack of fit had P-value > 0.51 [Table 2]. These values were fairly well aligned with the mathematical model. Besides, the correlation between experimental and predictable data is also shown in Figure 3.
 | Figure 3: Correlation between the experimentally and predict values for phenolics, tannin, flavonoid, vitamin C, 2,2-Diphenyl-1-picrylhydrazyl and FRAP. [Click here to view] |
3.3. Multiple Response Optimizations
Optimization is popular used method to find the best one among all the available alternatives. A lot of optimal techniques are utilized these days to easily understand and find the most suitable outcome. RSM is one of most frequently used experimental designs for optimization [33]. Moreover, RSM explores the relationships between several explanatory variables and one or more response variables [31]. The simultaneous optimization of multiple responses might be a main concern for industrial applications, especially that the energy cost of the process significantly diminished when extraction parameters are optimized [34,35]. Many scientists have used RSM to optimize research factors as Mundhe and Taralkar [36], Petrotos et al. [37], and Hong et al. [38]. The overplay plot showed the outlines superposition of all the studied responses and the simultaneous optimum for all responses was shown by the black spot [Figure 4]. The result showed that Hong Quan drink remained with high bioactive compounds and antioxidant capacity was 90.32oC and 25.05 min. At these optimal parameters, the content of phenolic, flavonoid, tannin, Vitamin C, DPPH, and FRAP were 28.903 mg GAE/100 g, 7.082 mg QE/100 g, 102.075 mg TAE/100 g, 15.728 mg/100 g, 85.771%, and 8.052 mM FeSO4/g, respectively.
 | Figure 4: Superposition plots showing the best experimental parameters that maximize bioactive compounds content and antioxidant capacity of product, the black spot shows the optimum for all the responses. [Click here to view] |
Furthermore, to validate the predicted values from the optimal models, the real test experiments have been conducted in same conditions of prediction model. In detail, the Hong Quan fruit drink was prepared and pasteurized at temperature of 90oC (90.32oC in the optimal model) in 25 min (25.05 min in the optimal model). The pasteurized fruit drink then was analyzed bioactive compounds such as phenolic, flavonoid, tannin, and Vitamin C, as well as the antioxidant activity through the assay of DPPH and FRAP. The results of real test sample were showed in Table 3 with three replications. In general, the behaviors of bioactive compounds in pasteurized drink were very similar with the predicted values of optimal models and were only differing from 0.79÷4.76%. In particular, phenolic and Vitamin C content was lower than predicted by 1.18% and 4.76%, while the flavonoid and tannin content were higher than predicted by 0.98% and 1.07%, respectively. The antioxidant activity of DPPH and FRAP were low difference percent of 0.79% and 0.86%. The content of Vitamin C has a high difference because optimal temperature and pasteurization time of this response factor was lower test condition. However, this difference is still within the allowable limit (±5%).
Table 3: Comparison of the predict values from the optimal model and test values.
| No. | Bioactive compounds | Test values* | Predict values | Difference percent |
|---|---|---|---|---|
| 1. | Phenolic (mgGAE/100 g) | 28.56±0.45 | 28.90 | 1.18 |
| 2. | Flavonoid (mgQE/100 g) | 7.15±0.27 | 7.08 | 0.98 |
| 3. | Tannin (mgTAE/100 g) | 103.18±1.21 | 102.08 | 1.07 |
| 4. | Vitamin C (mg/100 g) | 14.98±0.62 | 15.73 | 4.76 |
| 5. | DPPH (%) | 85.09±1.34 | 85.77 | 0.79 |
| 6. | FRAP (µMFeSO4/g) | 8.12±0.11 | 8.05 | 0.86 |
(*) Mean value (n=3) and SD (standard deviation)
4. CONCLUSION
The study found that the optimal pasteurization temperature and time of the ready-to-drink beverage from Hong Quan fruit was 90oC during 25 min. At these optimal pasteurization conditions, the fruit beverage maintained high levels of bioactive compounds including phenolic, flavonoid, tannin, and vitamin C content) and antioxidant activities (DPPH and FRAP assay). The product can be used as one of the beverages that have health benefit. In future, the product could become a potential functional beverage for customers.
5. ACKNOWLEDGMENTS
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number “C2021-16-03”.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All data generated and analyzed are included within this research article.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Huber, AK. Satisfaction, repurchase intent, and repurchase behavior:Investigating the moderating effect of customer characteristics. J Mark Res 2001;38(1):131-42. [CrossRef]
2. Norhayati H, Izzreen I, Hooi PT. Effects of pasteurization and different concentrations of xanthan gum on honey beverage. Food Res 2019;3:325-2. [CrossRef]
3. The healthy study group. A school-based intervention for diabetes risk reduction.N Engl J Med 2010;363:443-53. [CrossRef]
4. Biswas SC, Kumar P, Kumar R, Das S, Misra TK, Dey D. Nutritional composition and antioxidant properties of the wild edible fruits of Tripura, Northeast India. Sustainability 2022;14:12194. [CrossRef]
5. Yu J, Ahmedna M. Functional components of grape pomace:Their composition, biological properties and potential applications. Int J Food Sci Technol 2013;48:221-37. [CrossRef]
6. Baruah D, Neog B. Botanical, phytochemical and pharmacological review of Flacourtia jangomas (lour.) raeusch. Int J Curr Med Pharm Res 2016;2:244-7.
7. Jeyachandran R, Mahesh A. Enumeration of antidiabetic herbal flora of Tamil Nadu. Res J Med Plant 2007;1:144-8. https://doi.org/10.3923/rjmp.2007.144.148
8. Singh AK, Singh J. Evaluation of anti-diabetic potential of leaves and stem of Flacourtia jangomas in streptozotocin-induced diabetic rats. Indian J Pharmacol 2010;42:301-5. [CrossRef]
9. Parvin S, Kader A, Sarkar GC, Gosain SB. In-vitro studies of antibacterial and cytotoxic properties of Flacourtia jangomas. Int J Pharm Sci Res 2011;2:2786-90.
10. Aklima J, Mojumder S, Sikdar D. Total phenolic content, reducing power, antioxidative and anti-amylase activities of five Bangladeshi fruits. Int Food Res J 2014;21:119-24.
11. Dutta B, Borah N. Studies on nutraceutical properties of Flacourtia jangomas fruits in Assam, India. J Med Plants Stud 2017;5:50-3.
12. Sasi S, Nishat A, Tripathi YC. Ethnomedicinal, phytochemical and pharmacological aspects of Flacourtia jangomas:A review. Int J Pharm Pharm Sci 2018;10:9-15. [CrossRef]
13. Myers RH, Montgomery DC, Cook CM. Response Surface Methodology:Process and Product Optimization Using Designed Experiments. 3rd ed. New York:Wiley;2009.
14. Hossain MA, Al-Raqmi KA, Al-Mijizy ZH, Weli AM, Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed 2013;3:705-10. [CrossRef]
15. Eswari LM, Bharathi VR, Jayshree N. Preliminary phytochemical screening and heavy metal analysis of leaf extracts of Ziziphus oenoplia (L) mill. Gard. Int J Pharm Sci Drug Res 2013;5:38-40.
16. Laitonjam WS, Yumnam R, Asem SD, Wangkheirakpam SD. Evaluative and comparative study of biochemical, trace elements and antioxidant activity of Phlogacanthus pubinervius T. Anderson and Phlogacanthus jenkinsii C. B. Clarke leaves. Indian J Nat Prod Resour 2013;4:67-72.
17. Talreja T. Biochemical estimation of three primary metabolites from medicinally important plant Moringa oleifera. Int J Pharm Sci Rev Res 2011;7:186-8.
18. Adedapo AA, Jimoh FO, Afolayan AJ, Masika PJ. Antioxidant properties of the methanol extracts of the leaves and stems of Celtis Africana. Rec Nat Prod 2009;3:23-31.
19. Tola AB, Rufina AS, Adenike OO. Phytochemical analysis and antioxidant activities of ethanolic leaf extract of Brillantaisia patula. World J Pharm Res 2014;3:4914-24.
20. Argo BD, Amaliyah FA. Optimization of temperature and pasteurization time of soursop juice (Annona muricata) by response surface methodology in pilot scale. IOP Conf Ser Earth Environ Sci 2021;924:012043. [CrossRef]
21. Veláquez-Barreto FF, Tixe ER, Goicochea RC, Aliaga-Barrera I. Optimization of the functional properties of a drink based on tubers of purple mashua (Tropaeolum tuberosum Ruíz y Pavón). Agroind Sci 2020;10:63-70. [CrossRef]
22. Minatel IO, Borges CV, Ferreira MI, Gomez HA, Chen CO, Lima GP. Phenolic compounds:Functional properties, impact of processing and bioavailability. In:Soto-Hernandez M, editor. Phenolic Compounds Biological Activity. Vienna, Austria:IntechOpen;2017. [CrossRef]
23. Laslo V, Socaci S, Teu?dea A, Adrian T, Tofana M, Vicas SI. The effect of pasteurization time on phytochemical composition and antioxidant capacity of two apple cultivars juices. Bull Univ Agric Sci Vet Med Cluj Napoca Food Sci Technol 2018;75:67-77. [CrossRef]
24. Saini P, Singh P, Dubey S. Effects of processing time and temperature on the quality components of cape gooseberry and sweet lemon juice. Int J Pharm Pharm Res Hum J 2016;6:192-205.
25. Apak R, Gorinstein S, Bohm V, Schaich KM, Ozyurek M, Guclu K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC technical report). Pure Appl Chem2013;85:957-98. [CrossRef]
26. Gohari AR, Hajimehdipoor H, Saeidnia S, Ajani Y, Hadjiakhoondi A. Antioxidant activity of some medicinal species using FRAP assay. J Med Plants 2011;10:54-60.
27. Villano D, Fermandez-Pachon MS, Moya ML, Troncoso AM, Parilla GM. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007;71:230-5. [CrossRef]
28. Farahmand M, Golmakani MT, Mesbahi G, Farahnaky A. Investigating the effects of large-scale processing on phytochemicals and antioxidant activity of pomegranate juice. J Food Process Preserv 2017;41:e12792. [CrossRef]
29. Benjamin O, Gamrasni D. Microbial, nutritional, and organoleptic quality of pomegranate juice following high-pressure homogenization and low-temperature pasteurization. J Food Sci 2020;85:592-9. [CrossRef]
30. Zhang Q, Wu C, Fan G, Li T, Wen X. Characteristics and enhanced antioxidant activity of glycated Morchella esculenta protein isolate. Food Sci Technol (Campinas) 2018;38:126-33. [CrossRef]
31. Guan X, Yao H. Optimization of viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chem 2008;106:345-51. [CrossRef]
32. Zabeti M, Daud WM, Aroua MK. Optimization of the activity of CaO/Al2O3 catalyst for biodiesel production using response surface methodology. Appl Catal A Gen 2009;366:154-9. [CrossRef]
33. Reji M, Kumar R. Response surface methodology (RSM):An overview to analyze multivariate data. Indian J Microbiol Res 2022;9:241-8. [CrossRef]
34. Tsai CW, Tong LI, Wang CH. Optimization of multiple responses using data envelopment analysis and response surface methodology. Tamkang J Sci Eng 2010;13:197-203.
35. Spigno G, Tramelli L, De Faveri DM. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng 2007;81:200-8. [CrossRef]
36. Mundhe SB, Taralkar SV. Optimization of process condition to improve percentage purity of aloe emodin from Aloe vera by extraction using response surface methodology with the central composite design tool. Int J Health Sci 2022;6:10993-1007. [CrossRef]
37. Petrotos K, Giavasis I, Gerasopoulos K, Mitsagga C, Papaioannou C, Gkoutsidis P. Optimization of the vacuum microwave assisted extraction of the natural polyphenols and flavonoids from the raw solid waste of the pomegranate juice producing industry at industrial scale. Molecules2021;26:1033. [CrossRef]
38. Hong ZY, Chen LC, Li, YC, Hsu HL, Huang CM. Response surface methodology optimization in high-performance solid-state supercapattery cells using NiCo2S4-graphene hybrids. Molecules 2022;27:6867. [CrossRef]