1. INTRODUCTION
Nanofibers are commonly defined as fibers with diameters in the nanometer range. Nanofibers have outstanding mechanical characteristics which are acceptable and harmless in terms of biodegradability. Nanofibers have gotten a lot of interest recently because of its diverse application in energy storage and production, chemical and biological sensors, pharmaceutical and textile industries, water purification, and environmental remediation. Nanofibers may be created from a wide range of polymeric materials, which can be natural, synthetic, or a combination of the two. Natural polymers are less immunogenic and more biocompatible. Synthetic polymers, on the other hand, provide higher synthesis and modification flexibility [1]. Many synthetic and natural polymers have successfully been electrospun to make microfibers and, subsequently, nanofibers. Nanofibers are in great demand for application in biological research, industry, and the military due to the variety in fiber structure [2]. Drug delivery applications [3], tissue engineering, the textile sector, ballistic (clothing) protection, and wound dressing are all examples of this. Synthetic polymers, on the other hand, are generally not biocompatible. As a consequence, there is a strong need for nanofibers made from natural biopolymers that may be employed for biomedical and tissue engineering applications without creating hazardous effects [4].
Pectin is a biopolymer that occurs naturally and is increasingly employed in the food, pharmaceutical, and biotechnology sectors [5]. It is a water soluble naturally occurring biopolymer [6] which contains non-sugar substituents, particularly methanol, acetic acid, phenolic acids, and amide groups. Vegetables and fruits account for 45% of the yearly 30% food waste where the beverage business creates the largest food waste (26%), followed by the dairy and ice cream industry (21.3%) and the fruit and vegetable product and reserve (14.8%) [7]. Due to its biocompatibility and biodegradability, pectin has been used in several biological applications, including cell encapsulation, the generation of artificial red blood cells, bioprinting, and medication delivery [8]. As a natural product, pectin is more biodegradable and biocompatible than manufactured polymers [4]. Pectin has recently been studied as a material for the production of nanofibers. It is difficult to make nanofibers by electrospinning pectin alone [9]. Because of their high viscosity, synthetic polymers such as polyethylene oxide or polyvinyl alcohol (PVA) are mixed with pectin to increase its ability to be electrospun into fibers [10].
Citrus peel is the most prevalent source of pectin, followed by apple pulp, jackfruit, mango peel, and pomace [11], but this is to a lesser degree due to the sugar industry. Citrus fruit contains 13.4–29.1% pectin by dry weight, according to calculations [12]. When compared to extraction yields obtained using the acid isolation approach, enzymatic extraction revealed no significant difference in the recovery of pectin from lime peels, orange peels, and Citrus limon peels [13]. The second most widely used sources of pectin are apple pulps and pomace, which contain between 4.2% and 19% pectin and are high in neutral sugars such as glucose, arabinose, rhamnose, galactose, and xylose [14]. Citrus pectin produces a more elastic-brittle gel, while apple pectin produces a more elastic-viscous gel. Sugar beet pulp is also recognized as a good low-cost source of merchant pectin generated from the sugar industry. Reduced pH, increased temperature, extraction duration, and the solvent-solid relationship of the extraction process have all been connected to improved sugar beet pectin recovery rates ranging from 6.5% to 24.8% [15].
Electrospinning is a technique for creating nanoscale to microscale fibrous scaffolds by spinning of charged polymer solution with an electric force. This synthetic or natural polymer solution is jetted at a constant pace from a syringe through a small nozzle tip connected to a high voltage power source. As the droplet emerges from the tiny nozzle tip and interacts with the electric field, it deforms into an unstable conical shape known as a Taylor cone [4]. This means that the best feasible set of settings is being utilized. Deformation is caused by electrostatic repulsions on the droplet that develops on the nozzle tip. As the solution travels from the tip to the collecting plate, the organic solvent evaporates, and the Taylor cone divides into streams of nanofibers. When the technique is completed, a nanofibers scaffold is placed on the collecting plate [16].
The primary objective of this study is to extract pectin from C. limon (L.) Burm peels and transform the extracted pectin into nanofibers by electrospinning. PVA is used as a carrier polymer to incorporate into pectin solution for the fabrication of nanofibers. The nanofibers are characterized by means of X-ray diffractogram (XRD) and Fourier transfer infrared (FTIR). Electrospinning parameters such as voltage and flow rate are optimized to produce fibers of optimal quality for subsequent applications such as drug delivery [17] and production of extracellular matrix scaffolds [18].
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
Hydrochloric acid (HCL), sodium hydroxide, isopropanol, and pectin was provided by SRL diagnostics. Hot water soluble PVA (molecular weight −60,000–1,25,000) was provided by HiMedia. Fresh C. limon peel was collected from local market at Chennai, Tamil Nadu.
2.2. Extraction of Pectin from C. limon Peel
The pectin extraction began with cutting the fresh leftover C. limon peels from kitchen wastes and drying it before the experiment. After that, 40 g of fresh C. limon peels were cleaned and chopped into little pieces. Grounded C. limon peels were then treated for 2 h in a water bath at 80–90°C with 0.01 N HCL. The beaker was then removed from the water bath and allowed to cool. The pectin syrup is filtered through a clean washcloth to separate the peels and any large solid residues followed by filtration using Whatman no.1 filter paper to remove the pectin syrup. Filtration of pectin syrup was done thrice until a clear viscous solution was achieved which is then precipitated by adding a 1:2 ratio of isopropanol to the solution, which is then left undisturbed for 2 h. A distinct mark was seen in the solution, which was subsequently removed from the solution and dried in a vacuum oven at 400°C. After the leftover acid syrup was evaporated, the dry pectin powder was washed with distilled water to remove the acid residues and dried in the vacuum oven until all the water had evaporated and clean dry pectin powder was produced. Figure 1 depicts a schematic representation of the complete endeavor.
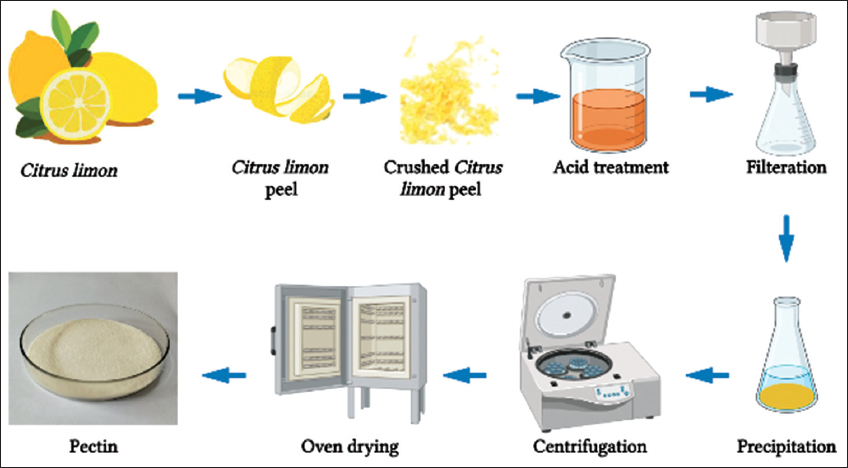 | Figure 1: Methodology flow. [Click here to view] |
2.3. Estimation of Pectin Content
The wet weight and dry weight of pectin were recorded when calculating the pectin for assessing the data for characterizing the pectin sample extracted. According to Salma et al., the yield (Eq. 1), moisture (Eq. 2), and ash (Eq. 3) content were estimated [19].
 |
After the estimation, the pectin obtained from the extraction of the C. limon peels was then tested and compared with the commercial pectin.
2.4. Preparation of the Solution for Electrospinning
The electrospinning setup included a syringe pump that slowly pushes the syringe to pump the Pectin/PVA solution, a 30 kV generator connected to the needle of the syringe to pump the electrically charged Pectin and PVA solution onto the collector, and a rotator covered in aluminum foil to collect the dried-up pectin and PVA nanofiber mats. The solution preparation parameters investigated includes 10 mL of pectin (5–10% w/v): PVA (5–10% w/v) dissolved separately in distilled water and blended together in a 50:50 ratio. The PVA was dissolved in distilled water at 90°C for 1 h while stirring continuously at 1200 rpm, while the pectin solution was made by adding the required quantity of distilled water and stirring at room temperature for 10 min at 1000 rpm. Mild stirring at 40°C for 2 h was used to combine the PVA and pectin solution.
The distance between the syringe needle tip and collector was designed to stay constant at 13 cm throughout the procedure. The flow rate was tuned to 0.5 mL/h–1 mL/h, and the voltage to 20 kV–25 kV. Each parameter was set to impact the others and explored by holding all parameters constant except for one variable [Table 2] [4].
Table 1: Pectin characterization.
| Characteristics | Extracted pectin |
|---|---|
| Total yield | 2% |
| Moisture content | 11.2% |
| Ash content | 3% |
Table 2: Parameters of all 4 samples.
| Samples | Combinations (%) | Flow rate | Voltage | Distance | ||
|---|---|---|---|---|---|---|
| PVA | Extracted pectin | Commercial pectin | ||||
| Sample 1 | 10 | 10 | - | 1 mL/h | 20 KV | 13 cm |
| Sample 2 | 10 | 10 | - | 1 mL/h | 25 KV | 13 cm |
| Sample 3 | 10 | 5 | - | 0.5 mL/h | 20 KV | 13 cm |
| Sample 4 | 10 | 5 | - | 0.5 mL/h | 25 KV | 13 cm |
| Sample 5 | 10 | - | 10 | 0.5 mL/h | 20 KV | 13 cm |
| Sample 6 | 10 | - | 5 | 0.5 mL/h | 25 KV | 13 cm |
2.5. Fabrication of Pectin Nanofiber
The syringes were loaded with 5 ml of the Pectin/PVA solution before being put in the syringe pump for a 5-h session. As soon as the voltage for the required parameter is applied to the needle, the syringe pump begins to push the syringe in accordance with the flow rate. The electrically charged pectin/PVA solution is then jetted onto the collector by the needle. The pectin/PVA nanofibers were subsequently electrospun onto an aluminum foil-covered rotator. After collecting the pectin and PVA nanofibers, the aluminum foil was removed from the rotator, and a thin coating of the pectin and PVA nanofiber mat was produced. Triplicates of the same combinations were run, and a tiny patch of the nanofiber mat generated on the aluminum foil was retrieved to be examined under the microscope, while the remaining samples were removed for further analysis.
2.6. Characterization of Pectin Nanofibers
The samples of pectin and PVA nanofibers from all electrospinning parameter settings were initially inspected under a ×40 microscope. The remaining fiber mat samples were also analyzed using FTIR spectroscopy.
The physical and chemical characteristics of the extracted pectin/PVA nanofiber were studied using several analytical techniques such as FT-IR and XRD. The Shimadzu IRTracer-100 spectrometer was used to analyze the physical, chemical, and conformational aspects of pectin/PVA since FT-IR analysis can describe numerous functional groups and structural fragments. For phase identification, XRD was used. The manufactured pectin/PVA nanofiber was scanned using a PANalytical’s X’pert X-ray diffractometer. The range of 2 for all samples was between 10° and 80°.
3. RESULTS AND DISCUSSION
3.1. Yield of Pectin
The total quantity of pectin extracted from fresh and dried C. limon peel varies depending on the precipitation agent used in this study (Isopropanol). Wet and dry pectin extracted from 40 g of C. limon peel is 0.9 g and 0.8 g in weight. The data shows that pectin production is 2%. According to our findings, the extracted pectin contained 11.2% moisture [Table 1], which is more than what is generally observed in commercial pectin. As a result, the extracted pectin must be stored in an airtight and dry atmosphere. In contrast to commercial pectin’s 15% ash content, we discover that the ash level of pectin extracted in our research is just 3% [Table 1], which might vary based on the citrus fruit and isolation techniques employed.
3.2. Pectin Nanofibers Electrospun
ESPIN-NANO was used to electrospin polymeric blends containing 5% w/v to 10% w/v PVA concentrations. When only pure pectin was utilized, no fibers were produced. When <5% w/v PVA was present in the total polymeric mix, no fiber was produced. The absence of fiber production might be attributed to low viscosity, which causes the polymer jet to break when stretched. The pectin content was raised to 5% by weight to lower the quantity of synthetic polymer utilized. Electrospinning samples with 5% pectin produced few droplets, indicating the importance of pectin concentration. The electrospun fibers produced with formulations including 5% PVA and 5% pectin (samples 3 and 4) at flowrates of 0.5 mL/h and voltages of 20 kV and 25 kV, as shown in Figure 2b-d, lacked the strength to be peeled off the aluminum foil collector in matrix format. When the PVA and pectin concentrations were increased to 10% w/v (samples 1 and 2), electrospinning was accelerated, resulting in the formation of nanofibers with improved stretchability, as shown in Figure 2a. Commercial pectin and PVA manufacturing resulted in superior nanofiber creation under all settings, with enhanced results at 10% w/v of each. Direct comparison of commercial pectin and extracted pectin with nanofibers revealed that the former produced superior outcomes. Figure 2e denotes the 10x microscopic image of the fabricated pectin/pva nanofiber.
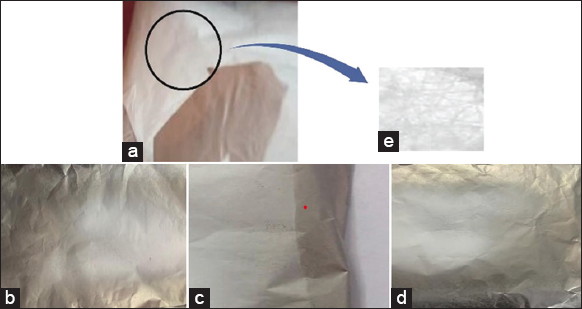 | Figure 2: Nanofiber mats fabricated using different parameters (a) Sample 1 (b) Sample 2 (c) Sample 3 (d) Sample 4 (e) Compound microscopic image of sample 1. [Click here to view] |
3.3. Characterization of Nanofibers
3.3.1. Spectroscopy FTIR
The FTIR spectra wavenumbers identified in Figure 3 confirm the peaks of commercial PVA and pectin/PVA nanocomposite explaining the structural and principle changes. The broad bands of the wavenumber are as follows: From left to right, the peak at 3292 cm-1 represent the O-H (hydroxyl) group expansion. The peak at 2937 cm-1 represents the stretching of C-H, 1724 cm-1 depict the carboxyl COO- group wherein the C=O of carbonyl is being stretched, 1653 cm-1 constitute the spectra of carboxylic group. The peak at 1425 cm-1 is a CH2 bond breakdown vibration [20].
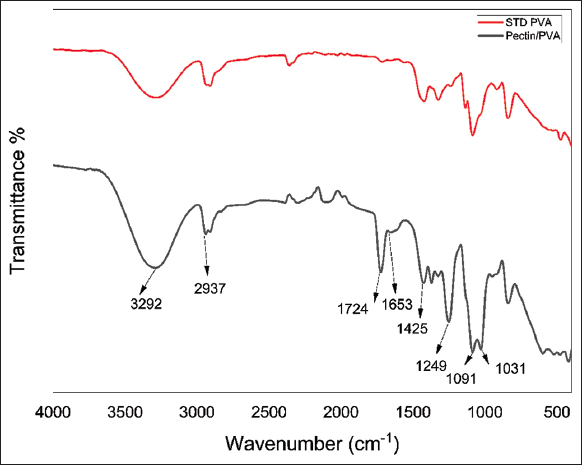 | Figure 3: FTIR spectra of standard PVA nanofiber and pectin/PVA nanofiber S1 (10%w/v each) nanofiber. [Click here to view] |
3.3.2. X-ray diffraction
XRD analysis was done to determine the nature (amorphous or crystalline) of the manufactured pectin, and the diffractogram is presented in Figure 4. Amorphous structures are generally identified in XRD patterns by a broad background peak, while crystalline structures are characterized by many sharp signals. The XRD pattern indicated that the pectin/PVA nanofiber was amorphous, and the presence of crystalline peaks at 65° and 75° indicates the peak of aluminum foil over which the fiber was formed [21].
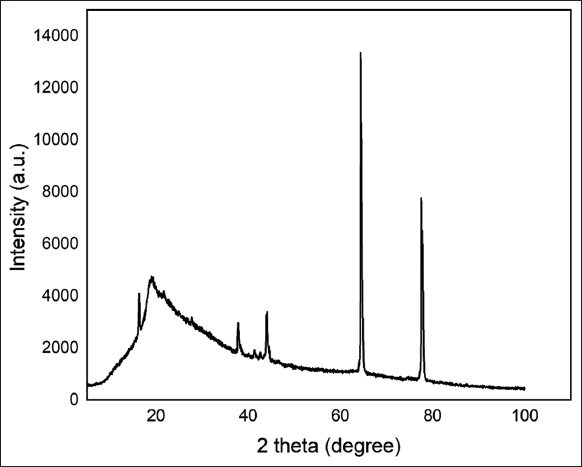 | Figure 4: X-ray diffractogram of S1 Pectin/PVA nanofiber. [Click here to view] |
4. CONCLUSION
The primary goal of this study was to develop a method for efficiently combining the advantageous features of a natural biopolymer with a synthetic polymer during processing, thereby creating nanofiber as a polymeric scaffold. To prevent droplet formation and maintain smooth needle-to-solution flow, the molecular concentration and volume ratio of polymers have been fine-tuned. Analytical methods have been used to characterize the performance differences between the polymers. To sum up, pectin and biopolymer like PVA can be combined simply to create 3D electrospun nanofibers. In the medical field, this pectin/PVA electrospun composites might find use as wound dressings or in tissue engineering. Although commercial pectin appeared to have better properties than extracted pectin, the productive utilization of biomass to produce useful products will be greatly appreciated as a waste-to-wealth application.
5. ACKNOWLEDGMENT
We are grateful to the Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur, for the financial and infrastructure support to carry out the research work.
6. AUTHORS CONTRIBUTION
GA, NH, PSY, KNKB, and RR have equally contributed in the execution of the experiment, data generation, data analysis, and drafting of the manuscript. RJ involved in the planning of the experiment and evaluation of the manuscript.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
The research work does not include experimentations on animals or human subjects.
9. DATA AVAILABILITY
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Wang F, Hu S, Jia Q, Zhang L. Advances in electrospinning of natural biomaterials for wound dressing. J Nanomater 2020;2020:1-14. [CrossRef]
2. Keshvardoostchokami M, Majidi SS, Huo P, Ramachandran R, Chen M, Liu B. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials (Basel) 2020;11:21. [CrossRef]
3. Induru J. Pectin-based nanomaterials in drug delivery applications. In:Biopolymer-based Nanomaterials in Drug Delivery and Biomedical Applications. Netherlands:Elsevier Science;2021. 87-117. [CrossRef]
4. McCune D, Guo X, Shi T, Stealey S, Antrobus R, Kaltchev M, et al. Electrospinning pectin-based nanofibers:A parametric and cross-linker study. Appl Nanosci 2018;8:33-40. [CrossRef]
5. Jong SH, Abdullah N, Muhammad N. Optimization of low-methoxyl pectin extraction from durian rinds and its physicochemical characterization. Carbohydr Polym Technol Appl 2023;5:100263. [CrossRef]
6. Lara-Espinoza C, Carvajal-Millán E, Balandrán-Quintana R, López-Franco Y, Rascón-Chu A. Pectin and pectin-based composite materials:Beyond food texture. Molecules 2018;23:942. [CrossRef]
7. Belkheiri A, Forouhar A, Ursu AV, Dubessay P, Pierre G, Delattre C, et al. Extraction, characterization, and applications of pectins from plant by-products. Appl Sci 2021;11:6596. [CrossRef]
8. Banks A, Guo X, Chen J, Kumpaty S, Zhang W. Novel bioprinting method using a pectin based bioink. Technol Health Care 2017;25:651-5. [CrossRef]
9. Zheng J, Yang Q, Shi X, Xie Z, Hu J, Liu Y. Effects of preparation parameters on the properties of the crosslinked pectin nanofiber mats. Carbohydr Polym 2021;269:118314. [CrossRef]
10. Serio F, da Cruz AF, Chandra A, Nobile C, Rossi GR, D'Amone E, et al. Electrospun polyvinyl-alcohol/gum Arabic nanofibers:Biomimetic platform for in vitro cell growth and cancer nanomedicine delivery. Int J Biol Macromol 2021;188:764-73. [CrossRef]
11. Karim R, Nahar K, Zohora FT, Islam MM, Bhuiyan RH, Jahan MS, et al. Pectin from lemon and mango peel:Extraction, characterisation and application in biodegradable film. Carbohydr Polym Technol Appl 2022;4:100258. [CrossRef]
12. Sundarraj AA, Ranganathan TV. A review-pectin from agro and industrial waste. Int J Appl Environ Sci 2017;12:1777-801.
13. Methacanon P, Krongsin J, Gamonpilas C. Pomelo (Citrus maxima) pectin:Effects of extraction parameters and its properties. Food Hydrocoll 2014;35:383-91. [CrossRef]
14. Robledo VR, Castro Vázquez LI. Pectin - Extraction, Purification, Characterization and Applications. Martin Masuelli:Rijeka;IntechOpen 2019.
15. Abou-Elseoud WS, Hassan EA, Hassan ML. Extraction of pectin from sugar beet pulp by Enzymatic and ultrasound-assisted treatments. Carbohydr Polym Technol Appl 2021;2:100042. [CrossRef]
16. Anusiya G, Jaiganesh R. A review on fabrication methods of nanofibers and a special focus on application of cellulose nanofibers. Carbohydr Polym Technol Appl 2022;4:100262. [CrossRef]
17. Kiadeh SZ, Ghaee A, Farokhi M, Nourmohammadi J, Bahi A, Ko FK. Electrospun pectin/modified copper-based metal-organic framework (MOF) nanofibers as a drug delivery system. Int J Biol Macromol 2021;173:351-65. [CrossRef]
18. Hosseini SA, Hoseini SJ, Askari VR, Salarinia R, Ebrahimzadeh-Bideskan A, Tara F, et al. Pectin-reinforced electrospun nanofibers:Fabrication and characterization of highly biocompatible mats for wound healing applications. J Drug Deliv Sci Technol 2022;77:103916. [CrossRef]
19. Salma MA, Jahan N, Islam MA, Hoque MM. Extraction of Pectin from lemon peel:Technology development. J Chem Eng 2014;27:25-30. [CrossRef]
20. Patra N, Salerno M, Cernik M Electrospun polyvinyl alcohol/pectin composite nanofibers. In:Electrospun Nanofibers. Netherlands:Elsevier Science;2017. 599-608. [CrossRef]
21. Sen S, Bal T, Rajora AD. Green nanofiber mat from HLM-PVA-Pectin (Hibiscus leaves mucilage-polyvinyl alcohol-pectin) polymeric blend using electrospinning technique as a novel material in wound-healing process. Appl Nanosci 2022;12:237-50. [CrossRef]