1. INTRODUCTION
After first appearing in China in late 2019, the severe acute respiratory syndrome coronavirus 2 disease (SARS-CoV-2) known as COVID-19 caused a significant global outbreak and public health issue [1]. Globally, weekly new cases in the week of 5–11 December 2022 remained stable (+2%) compared to the previous week, with over 3.3 million new cases reported. The number of new deaths each week increased by more than 10% compared to the previous week, with over 9700 new deaths reported. On December 11, 2022, it had over 645 million confirmed cases and more than 6.6 million deaths worldwide [2]. This disease is caused by two coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21, which are closely related to bats [3]. The virus was initially referred to as the novel coronavirus (2019), later renamed SARS-CoV-2 [4]. The virus is thought to have an average incubation period of 6.4 days and spreads through droplets or direct contact [5]. Fever, coughing, and the presence of Ground-glass opacity on chest computed tomography examination were the most frequently reported symptoms [3]. Until now, several variants are still infecting various countries, including alpha (first country detected in United Kingdom), beta (First country detected in South Africa), gamma and zeta (First country detected in Brazil), epsilon (First country detected in USA), eta (First country detected in Nigeria), theta (First country detected in The Philippines), iota (First country detected in USA), delta and kappa (First country detected in India), lambda (First country detected in Peru), mu (First country detected in Colombia) dan omicron (first country detected in Botswana, Germany, South Africa, and India) [6].
Although remdesivir [7,8] and glucocorticoids have shown promise in treating COVID-19 [9], there are few effective treatments available. Various efforts have been made to anticipate this pandemic, including preventing the spread of the virus and vaccine development. Although SARS-CoV-2 is currently the subject of a preventive or curative vaccine, research into this virus must still be done to control the outbreak better. One of the research targets is to study various pathophysiological processes that arise in COVID-19. Acute respiratory distress syndrome (ARDS), a severe condition occurring after direct or indirect lung injury, is one type of the syndrome in COVID-19. There is no definitive pharmacological therapy; supportive care is the cornerstone of treatment.
In patients infected with COVID-19, there is often an increase in elastase levels in the airways, so an alternative is needed to reduce elastase levels. Several natural product compounds have been shown to have activity as elastase inhibitors, although so far, they have only limited in silico, in vitro, and in vivo tests. Therefore, it is fascinating to conduct a more in-depth study to prove the potential of natural ingredients as elastase inhibitors in handling COVID-19.
Elastase is a proteolytic enzyme found in smooth muscle tissue, particularly in the lungs. In respiratory diseases with a marked inflammatory response, the ratio of proteolytic enzymes to their inhibitors is frequently not neutral. In several clinical conditions, increased elastase in COVID-19 patients damages tissue and interferes with the remodeling process [10,11]. However, since neutrophils are essential for the innate immune response, they interact in the protection against infections. Serine proteases (proteinase 3, cathepsin G, and elastase), which are proteolytically active enzymes that can decompose a wide range of extracellular matrix proteins, including fibronectin, elastin or collagen, which give tissues physical support and stability, are released into the extracellular space as a result of neutrophil activation and degranulation. Elastase is one of the neutrophil-derived proteases that can regulate the activity of inflammatory cytokines by triggering the immune system [12-15].
Therefore, it can be viewed as an alternative in terms of treatment and an attempt to provide some background to inform future clinical guidance. In addition, it is hoped that using natural product resources in conjunction with a sustainable research methodology and referring to current research findings as “elastase inhibitors” will help combat these infectious diseases. This paper aims to comprehensively examine the elastase and COVID-19 relationship and potential natural resource as elastase inhibitors.
2. MATERIALS AND METHODS
In this review study, the literature was searched with an online engine machine system, including Google Scholar (https://scholar.google.com/), Directory Open-Access Journal (DOAJ, https://doaj.org/), National Health Institute (NIH, https://www.nih.gov/), Pubmed (https://pubmed.ncbi.nlm.nih.gov/), and ScienceDirect (https://www.sciencedirect.com/) between October 2021 and August 2022. The data used in the review were all taken from English-language articles. Different keywords were used: elastase, COVID-19, neutrophil elastase (NE), NE trap, inflammation, cytokine storm, natural product, and polyphenols.
3. RESULTS AND DISCUSSION
3.1. General Pathophysiology of COVID-19
In the first pathophysiology, when a pathogen enters the body, cytokines signal T cells and macrophages to go to the infected area and produce more cytokines. High-pathogenic levels are conditions of excessive cytokine production and the potential for a cytokine storm to occur, which can occur if an excessive cytokine storm can damage tissues and organs. The second pathophysiology, interleukin-6 (IL-6), induces endothelial cells that cause dysfunction and coagulopathy in COVID-19, inactivates endothelial nitric oxide synthase enzyme resulting in nitric oxide (NO) production, and oxidative stress causes a decrease in endothelial response. The third pathophysiology is a decrease in lymphocytes, susceptible to secondary infections. Secondary infections are repeated infections caused by different pathogens due to reduced immune cell components. Inflammation in COVID-19 is caused by the neutrophil response, which contains numerous protease enzymes such as elastase, proteinase-3, and cathepsin G, which will be inhibited by severe lymphopenia [16]. Elastase is a neutrophil enzyme reported to increase excessively in patients with COVID-19 [17].
3.2. Cytokine Storms Impact the Respiratory System
The pathology of pneumonia due to the COVID-19 virus causes the deregulation of inflammation in the lungs, which often leads to complete respiratory failure and sometimes death. Diffuse alveolar damage and severe hypoxia associated with this respiratory failure necessitate oxygen therapy through artificial ventilation in the hospital’s intensive care unit [18,19].
In COVID-19, lethal cytokines are released (“cytokine storm”). Neutrophils are thought to be essential in hyperinflammation, leading to acute lung injury and even irreversible lung tissue degradation [20], as shown in Figure 1. The greatest threat comes from the innate immune system’s overreaction rather than the SARS-CoV-2 virus itself [21]. Neutrophils produce NETs, proteases, and pro-inflammatory cytokines, particularly elastase-associated serine proteases, whose proteolytic activity results in acute lung injury and exacerbate inflammation [21].
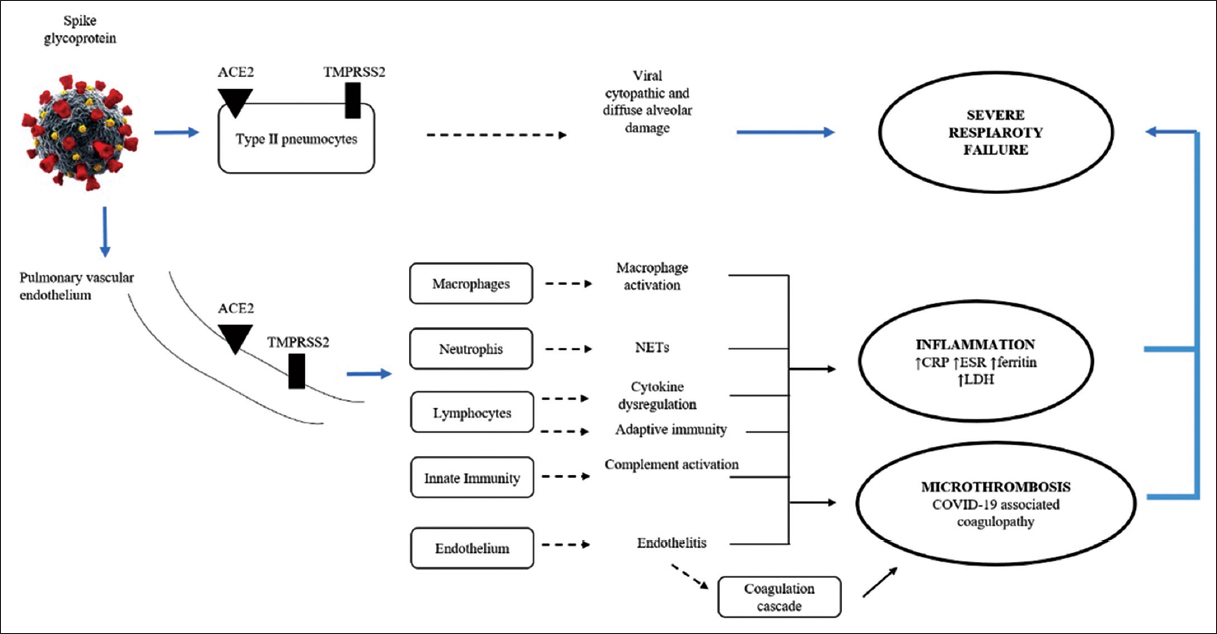 | Figure 1: Direct and indirect injury to the lungs by SARS-CoV-2 involves interactions between coagulation and inflammatory pathways. ACE2, ESR erythrocyte sedimentation rate, CRP C-reactive protein, ESR erythrocyte sedimentation rate, NETS neutrophil extracellular traps, LDH lactate dehydrogenase, SARS-COV-2, TMPRSS2 transmembrane protease serine 2 [20]. [Click here to view] |
When the body is infected with a virus, it activates an antiviral immune response. There are two types of antiviral immune response: Innate and adaptive immune response [22,23]. These two responses will interact with each other to produce immune protection but have different limits and times of action. The innate immune response occurs after infection occurs and is relatively weak in clearing the virus [24]. Meanwhile, the adaptive immune response usually begins 4–7 days after infection and is crucial in destroying the virus beforehand. However, suppose the body does not generate an effective adaptive antiviral response in time to clear the virus. In that case, the innate immune response will be amplified, which cannot eliminate the virus effectively and instead causes a systematic inflammatory response with uncontrolled release of inflammatory cytokines [25]. Recent studies have shown that the average age of severely and critically ill patients is higher than that of mild cases.
In contrast, for elderly patients and those with chronic illnesses, it takes longer to generate an effective adaptive immune response due to decreased immune function [26,27]. These patients rely solely on strengthening the innate immune response in the early stages of infection, leading to a higher risk of cytokine storms, earlier onset of severe disease, and higher death rates. Rapid viral replication leading to increased pyroptosis can lead to the massive release of inflammatory mediators [28]. Taken together, viral shedding to evade antiviral immunity and genetic or acquired defects in host defense can impair viral clearance, resulting in inappropriate immune activation and consequent cytokine storms. In summary, the over-activation of innate immunity is essential in forming cytokine storms in COVID-19 patients [29].
3.3. Protease Enzymes and Protease Inhibitors
Chronic obstructive pulmonary disease (COPD), one of the leading causes of mortality and morbidity in the world, is becoming more prevalent. One characteristic of COPD is the existence of partially reversible airflow obstruction. This pathology is connected to airway inflammation, characterized by an overabundance of inflammatory cells such as neutrophils and macrophages and various pro-inflammatory cytokines secretion, including TNF-α, IL-1β, IL-6, IL-8, and IL-17 [30]. The development of emphysema indicates the presence of excess protease enzymes that will damage the lung connective tissue and reduce the antiproteolytic ability to protect it. The “protease-antiprotease imbalance” hypothesis, which is frequently used to describe this theory, primarily concerns serine proteases like NE and matrix metalloproteinases (MMPs) [31]. MMPs is an enzyme capable of degrading many pericellular substrates and is closely associated with many inflammatory diseases [32,33]. One type of MMPs is MMPs-12 (elastase) which is one of the leading causes of elastin degradation [33]. MMPs-12 (macrophage elastase), which is involved in tissue repair procedures, can regulate extracellular matrix components of elastin [34].
SARS-CoV is affected by protease enzymes such as trypsin and elastase. The presence of proteases will increase the entry of the virus from the cell surface so that the infection will increase [35]. In lung inflammation, neutrophils will produce high elastase. Tests on mice developed fatal pneumonia, a condition exacerbated by coinfection with SARS-CoV (highly pathogenic similarity to human SARS) and non-pathogenic respiratory bacteria that stimulate elastase secretion [36,37]. Severe rat pneumonia is associated with increased SARS-CoV lung infection, which can also be facilitated/mediated by elastase [38,39] also hypothesized the importance of the presence of a protease inhibitor, namely, alpha-1-antitrypsin. This molecule has many biological activities that can fight several pathophysiological mechanisms caused by the SARS-CoV-2 virus. This substance is one of the promising agents to improve the condition of COVID-19 patients [27].
3.4. Neutrophil Extracellular Traps (NETs)
NETs are proteins released extracellularly from activated neutrophils in response to infection. NET can prevent the spread of microbes in the blood by trapping them mechanically and utilizing the function of coagulants to separate them in circulation, as well as triggering anti-inflammatory processes [38]. Patients with COVID-19 have an increased number of NETs, which can lead to lung hyperinflammation and respiratory failure. The NET reduction can lower the risk of SARS-CoV-2-related death [40]. When infection occurs, neutrophils are recruited to kill pathogens by phagocytosis and oxidative processes [41,42]. There are several theories that neutrophils kill pathogens by forming NETs. NE, peptidyl arginine deiminase type 4 (PAD4), and gasdermin D are enzymes involved in the development of NET [43-47]. NET is very useful in defense against pathogens; damage in NET formation will impact the development of diseases, including viral infections [41].
3.5. NETs Cell Death
NETs cell death or NETosis occurs due to intracellular oxidative bursts caused by viral infection. When neutrophil NETosis releases DNA and proteins into the extracellular space, a spider web-like structure is formed [40]. Innate immunity activates the receptors Nox2, MPO, NE, and calcium-dependent protein-arginine deiminase type 4 to cause NET formation (PAD4) [40].
Chromatin decondensed as a result of PAD4 citrulline and histones reducing the positive charge by changing the positive direction of arginine to neutral citrulline. Patients with mutations in Nox2 who have the chronic granulomatous disease have neutrophils that do not produce oxygen and do not cause NET formation [40,48,49]. Results of studies on plasma isolated from autoimmune patients, NET formation involving Nox2, and MPO (myeloperoxidase) [40].
3.6. Elastin and Desmosine
Elastin is an essential protein in maintaining the elasticity of tissues to keep them firm and supple [50,51]. Elastin is synthesized by fibroblasts and keratinocytes and is found in several connective tissues in blood vessels, lungs, and skin. Elastin is essential for elastic recoil of the small airways, makes up about 2.5% of the dry weight of the lungs, and is widely distributed throughout the lungs [52,53]. In the case of pulmonary emphysema, the elastin from the lung parenchyma will decrease so that the elastic fibers become irregular and do not function properly [54]. As already explained, this elastin is broken down by a protease enzyme, elastase [55].
The breakdown of elastin results in desmosine. Desmosine levels rise in people with COPD and rise in direct proportion to the rate at which lung function declines [56]. Alveolar macrophages from COPD patients demonstrated a more remarkable capacity for elastin degradation in in vitro tests than macrophages from healthy volunteers. An important mediator in the pathogenesis of both acute lung injury (pulmonary fibrosis) and chronic lung injury is MMP-12’s involvement in inflammatory lung disease (emphysema) [31]. Plasma levels of desmosine and isodesmosine are commonly used as markers for the rate of elastin degradation. Desmosine levels were significantly higher in Covid-19 patients (0.38 ng/L) compared to controls who had never smoked (0.24 ng/L) and long-time smokers (0.28 ng/L). COVID-19 patients with poor conditions also showed higher desmosine levels than patients with good conditions [57].
3.7. Elastase and Anti-elastase
Patients with various underlying diseases and injuries can experience acute severe hypoxia in the case of ARDS. Various therapeutic endeavors have been undertaken to utilize inhaled NO, neuromuscular blocking medications, and anti-inflammatory corticosteroids. According to the ARDS pathological study, non-hydrostatic pulmonary edema was caused by neutrophils’ predominant uncontrolled inflammation, alveolar epithelial cell layers, and the pulmonary microvascular endothelium having increased permeability. A NE inhibitor is used for this. Neutrophils produce the protease enzyme NE. Its primary physiological function is to degrade phagocytosed foreign organic molecules in the cell. Elastin, collagen, pulmonary surfactant, and immunoglobulins are just a few of the extracellular proteins that can be broken down by neutrophil extracellular elastase, an extremely destructive enzyme. Sivelestat is one instance [58-61].
In a non-clinical study, sivelestat was found to help treat acute lung injury without impairing the body’s immune response to infection. Clinical research has not produced a definite consensus. Improved mechanical ventilation, a shorter stay in the intensive care unit, and improved lung function were all seen in phase III and phase IV trials. However, other multicenter studies did not demonstrate how sivelestat affected the number of days without a ventilator. The length of stay, mechanical ventilation, arterial oxygen partial pressure ratio, and inspired oxygen fraction (PaO2) all showed improvement, and there were no adverse effects or information on infection exacerbation. Compared to standard care, the cost-effectiveness analysis indicated that sivelestat therapy could lower costs. Generations of powerful NE are new [62-65].
3.8. Elastase and Thrombin
It has been found that 30.6% of hospitalized COVID-19 patients had antithrombin levels below average, and of them, 66.7% died. Furthermore, 80% of patients with low antithrombin who survive require mechanical ventilation. According to data, high mortality rates are associated with low antithrombin levels; heparin’s ineffective anticoagulation action may cause this association. For COVID-19 patients, thrombosis (venous and arterial) is the most common cause of death; among all deceased patients, microthrombi were found in the pulmonary arteries [66]. An overly procoagulant state brought on by excessive inflammation is what causes thromboembolism. Antiphospholipid antibodies, abnormal coagulation, and lupus anticoagulant are all present in patients with severe COVID-19 [67]. Thromboembolism is treated with prophylactic or anticoagulation therapy for COVID-19, using heparin. Heparin will interact with antithrombin.
Nevertheless, severe cases of inflammation can reduce antithrombin synthesis, so that antithrombin levels are reduced. Inflammation also decreases elastase by activating neutrophils and thrombin formation. Antithrombin function also depends on glycosaminoglycans present on the endothelial surface, where levels are reduced [66,68].
3.9. Elastase and angiotensin-converting enzyme 2 (ACE2)
The immune response, inflammatory responses, circulatory dysfunction, and hypoxia are all indirect causes of COVID-19 pathology and direct viral infection of target cells. The heart contains cardiofibroblasts, cardiomyocytes, endothelial cells, pericytes, and epicardial adipose cells, which are home to ACE2. ACE2 binds to the spike proteins of SARS-CoV and SARS-CoV-2 in the vascular system [34]. For viral cell entry, transmembrane protease serine 2 (TMPRSS2) or other proteases’ S subunits are necessary (cathepsin L, cathepsin B, factor X, trypsin, elastase, and furin). Hence, elastase and ACE2 are enzymes the virus needs to form angiotensin 2 [69].
Due to its higher binding affinity to ACE2, SARS-CoV-2 has a higher transmission rate than SARS-CoV. Due to higher plasma ACE2 levels, people with cardiovascular disease frequently experience more severe COVID-19 disease [70]. However, the heart has a higher ACE2 and a lower TMPRSS2 content than the lungs. The lower level of ACE2 has some effect on reducing cardiac susceptibility to SARS-CoV-2 infection. The heart’s susceptibility to SARS-CoV-2 can be increased by other protein S priming protease enzymes such as cathepsin L and furin. In addition to viral toxicity mediated by ACE2, it can also be caused by an inability to regulate the RAAS (renin–angiotensin-aldosterone system), endothelial cell destruction and thrombo-inflammation, cytokine storms caused by a failure to control the body’s immune system, and a mismatch between oxygen availability and demand [70].
3.10. Elastase and Cathepsin C (Cat C)
Numerous results from preclinical and clinical studies show neutrophils’ essential role in acute lung injury by releasing serine proteases linked to elastase and reactive oxygen species. Numerous results from preclinical and clinical studies show neutrophils’ essential role in acute lung injury by releasing serine proteases linked to elastase and reactive oxygen species. One that can activate serine proteases such as elastase is Cat C. This cysteine dipeptidyl amino-peptidase activates most of the serine proteases associated with elastase that damages tissues. CatC protein acts by removing the N-terminal propeptide. Patients with a mutational disorder in the CatC gene are called Papillon-Lefèvre syndrome (PLS). In PLS patients, there were no immunodeficiency or recurrent viral infections. This is probably due to a decrease in protease-related pro-inflammatory elastase in neutrophils. Eliminating elastase-associated neutrophil proteases in these patients may retard the development of lung injury. To prevent tissue degradation caused by proteases in inflammatory diseases, CatC may be a potential therapeutic target [21].
Maladaptive cytokine release occurs in COVID-19 patients in response to infection and other stimuli. The development and progression of ARDS inflammation are closely correlated with this cytokine storm event. The pathogenesis is complicated, but one aspect is the loss of local and systemic regulatory control over the production of pro-inflammatory cytokines [71,72]. A growing problem for intensive care units due to the COVID-19 pandemic is ARDS, characterized by diffuse alveolar inflammation. Neutrophils may play a role in this. Neutrophil proteases associated with elastase may be eliminated to slow the development of lung damage in patients [21]. As a major inflammatory response to endothelial injury components, neutrophils have proteolytic and pro-apoptotic properties through several enzymes, including serine proteases and NE [73]. Pharmacological CatC inhibition is a potential therapeutic strategy to halt irreversible pulmonary failure putting the lives of COVID-19 patients in danger [21,71]. The pharmacological mechanism of action of CatC inhibition is illustrated in Figure 2.
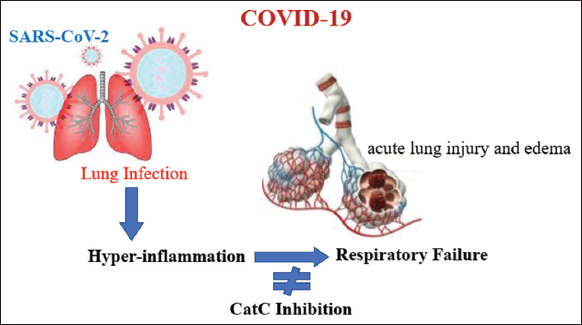 | Figure 2: The role of pro-inflammatory elastase-related protease in neutrophils with CatC deficiency [21]. [Click here to view] |
3.11. Correlation of Elastase and Infection with SARS-Cov-2 Virus
A robust immune response accompanies early infection, including changes in T and B cells, changes in how virus-specific lymphocytes develop, mitochondria, and oxidative stress. Neutrophils have a role in the blood and lungs, and changes in their quantity and phenotype indicate severe COVID-19 pathophysiology. Neutrophils facilitate the proteolytic degradation of tissue elasticity by excessively releasing virus-induced extracellular traps (NETs). Furthermore, NETs, which function as additional innate immune system defenses, can be produced by interacting with chromatin threads and peptides derived from granules. Acute hemorrhagic or thrombotic plaque complications are also brought on by the NE/DNA complex of the NET [63]. As a result, intravascular NET accumulation in COVID-19 patients with the severe form of the disease interferes with the plasminogen proteolytic pathway, leading to platelet trapping, fibrinolytic collapse, and microvascular occlusion, which ultimately leads to multi-organ failure. NET, which directly kills epithelial and endothelial cells, has also been shown to have the ability to harm tissue [74].
In COVID-19 patients, plasma levels of NE, elastase, IL-6, and IL-8 in patients experienced a significant increase compared to controls [63]. Two biomarkers for elastin degradation, desmosine and isodesmosine (DES), were also significantly elevated in patients. The correlation between DES levels and IL-6 levels in COVID-19 patients suggests a direct link between inflammation and lung/vascular tissue damage. It can be assumed that elastase may destroy the extracellular matrix components of the lung parenchyma, including collagen, elastin, glycosaminoglycans, and fibronectin, and that elastase may also encourage pulmonary fibrosis. In addition, NE can increase Notch1 expression, which causes alveolar epithelial cells to differentiate into myofibroblasts through the TGF-Smad3 signaling pathway.
Furthermore, Notch1 activation directly increases the expression of smooth muscle actin (SMA) to support myofibroblast differentiation. Numerous studies have shown that NE, TGF-, and -SMA are markedly upregulated in COVID-19 patients. In addition, ROS is a significant signal that can cause NETosis. In particular, myeloperoxidase (MPO) is stimulated by ROS produced by NADPH oxidase, allowing NE to be released from azurosomes found in granules. After that, NE moves into the nucleus and contributes to the chromatin’s proteolytic disruption necessary for NET formation. The fact that NE is regarded as an independent predictor of multi-organ damage in COVID-19 patients, as opposed to histone-DNA and MPO-DNA, emphasizes the significance of elastase in SARS-CoV-2 infection [74].
The production of antiviral cytokines, chemokines, and antimicrobial peptides by neutrophils that invade the lung during viral infection is thought to aid the ongoing antiviral response. Neutrophils are phagocytes that can remove pathogens and debris. These processes can help with viral control, viral clearance, and inflammation resolution. By overproducing MPO, NE, MMPs, oxidative stress, and NETs, an excess of activated neutrophils can cause lung tissue damage and exacerbate disease severity [75].
3.12. COVID-19 Therapy
The most common therapy is hydroxychloroquine (HCQ) which has anti-inflammatory and immunomodulatory effects. Drugs such as corticosteroids, tocilizumab, the macrolide azithromycin, the antiviral lopinavir/ritonavir, and the anticoagulant enoxaparin can all be combined with HCQ or taken alone. However, this HCQ was found to increase the risk of cardiac arrhythmias and is no longer allowed by the FDA for use, detailed COVID-19 therapy as shown in Table 1.
Table 1: The potential drugs for COVID-19 therapy.
| S. No | Drugs | Mechanism | References |
|---|---|---|---|
| 1 | Apixaban | Anticoagulation | [76] |
| 2 | Arbidol | Inhibiting the synthesis of viral DNA or RNA | [77] |
| 3 | Azithromycin | IL-6 Inhibitor | [78] |
| 4 | BDB-001 injection | Monoclonal antibody drug targeting human C5a molecule | [77] |
| 5 | CAStem cell injection | Secreting immunomodulatory factors, alleviate pulmonary inflammation | [77] |
| 6 | Convalescent plasma product | Passive immunity, immediately obtained after infusion | [77] |
| 7 | Corticosteroids | Impaired virus clearance, stimulate the massive production, and release of activated immune cells | [79] |
| 8 | Enoxaparin | Anticoagulation | [76] |
| 9 | Favipiravir | RdRp inhibitor | [77] |
| 10 | Glucocorticoid | Hormone regulating stress response, anti-inflammatory, and immunosuppressant | [77] |
| 11 | Heparin | Anticoagulation | [76] |
| 12 | Hydroxychloroquine (HCQ) | Preventing the replication of DNA or the transcription of RNA | [80] |
| 13 | Lactoferrin | Direct interaction with virus surface, DNA, or cell surfaces | [81] |
| 14 | Lopinavir/Ritonavir | Increasing the antiretroviral activity against the virus by inhibiting cytochrome P450 | [77,82] |
| 15 | Remdesivir | RdRp inhibitor, nucleotide analog prodrug | [77] |
| 16 | Ribavirin | Antiretroviral nucleoside drug | [77] |
| 17 | Sivelestat sodium | Selectively inhibiting the release of elastase by neutrophils | [77] |
| 18 | Tocilizumab | Monoclonal antibody against human IL-6 receptor | [77] |
Lactoferrin has an immunomodulatory, anti-inflammatory effect and can occupy the ACE-2 receptor as the only receptor for the SARS-CoV-2 virus. Several studies have shown that destroying the neutral endopeptidase enzyme can cause further pathophysiological changes. Increased neutrophil recruitment, activation, and vascular permeability are to blame for this. The release of NE enzymes like cathepsin G as a result of neutrophil chemotaxis damages airway tissue and aggravates the condition [16].
Small molecule agents and biological products are among the new therapeutics that have undergone clinical trials in China. Products classified as biologicals include stem cell therapy and human C5 monoclonal antibodies. Agents with small molecules include sodium sivelestat, remdesivir, and favipiravir. With broad-spectrum anti-influenza activity, favipiravir (Avigan®) is a medication that inhibits RdRp. With a similar mechanism of action to favipiravir, the nucleotide analog prodrug remdesivir can inhibit RdRp. An anti-inflammatory medication called sivelestat sodium treats acute lung injury brought on by SIRS. Sivelestat sodium can pass through the opening between neutrophils and tissues thanks to its low molecular weight. The ability of sodium sivelestat to inhibit enzymes without being harmed by reactive oxygen also makes it effective at inhibiting NE in inflammatory conditions. Sivelestat sodium can enhance lung injury brought on by SIRS and idiopathic pulmonary fibrosis, decrease the need for respirators and their associated risks of respiratory injuries and infections, and improve respiratory function [76].
3.13. Natural products resource as elastase inhibitors
Studies on elastase have been carried out over the last three decades and have become the object of extensive research in developing efficient elastase inhibitors. It is well known that increased recruitment and activation of NE is involved in the pathogenesis of many autoimmune and inflammatory diseases in humans, such as aging, rheumatoid arthritis, and systemic lupus erythematosus COPD (including COVID-19) [83-85]. The medicinal chemistry community has actively researched and developed human NE inhibitors. Most research has focused on developing synthetic elastase inhibitors with high potency, especially against unwanted side effects. Several natural or synthetic elastase inhibitors have been used to treat diseases related to human NE [86].
Exploration of natural products, mainly plants, has become a research target as a potential source of an efficient elastase inhibitor. However, it is still limited to exploring the potential of elastase inhibitors in vitro and in vivo [87]. On the other hand, the previous studies investigating the inhibition of HNE by secondary metabolites did not include a detailed kinetic characterization. In addition, there are still few studies on the isolation and determination of the structure of active compounds from natural ingredients that target this vital enzyme. Therefore, some research results on exploring plants as elastase inhibitors are presented in Table 2.
Table 2: The potency of natural products from plants as an inhibitor elastase.
| S. No | Scientific Name | Family | Part of plants | Elastase inhibition activity | References |
|---|---|---|---|---|---|
| 1 | Sterculia quadrifida R. Br | Sterculiaceae | Whole plant | 73.7 µg/mL | [88] |
| 2 | Callistemon lanceolatus | Myrtaceae | Stems | 1.5 µg/mL | [89] |
| 3 | Aspilia helianthoides (Schumach. and Thonn.) | Asteraceae | Leaves | 0.4-8.5 µg/mL | [90] |
| 4 | Ceropegia rupicola Defl. | Apocynaceae | Whole plant | 6.6-9.5 µg/mL | [90] |
| 5 | Kniphofia sumarae Defl. | Liliaceae | Whole plant | 1.6-79.4 µg/mL | [90] |
| 6 | Pavetta longiflora Vahl. | Rubiaceae | Leaves | 3.1-55 µg/mL | [90] |
| 7 | Plecatranthus cf barbatus (Thulin and Gifri) | Lamiaceae | Leaves | 1.6-10 µg/mL | [90] |
| 8 | Alchornea cordifolia (Schum. And Thonn) | Euphorbiaceae | Leaves | 2.2 mg/L | [91] |
| 9 | Vitis vinifera L. | Vitaceae | Grape | 14.7 µg/mL | [92] |
| 10 | Spatholobus suberectus | Fabaceae | Stems | 1.33 µg/mL | [93] |
| 11 | Rubus fraxinifolius | Rosaceae | Stem | 128. 85 µg/mL) | [94] |
| 12 | Stenocarpus sinuatus | Proteaceae | Leaves | 177.5 µg/mL | [95] |
| 13 | Melaleuca styphelioides | Myrtaceae | Leaves | 2,51 µM | [96] |
| 14 | Dodonaea viscosa (L.) Jacq | Sapindaceae | 2.4 µM | [97] | |
| 15 | Campylotropis hertella | Leguminosae | Roots | 8.5–30.8 µM | [98] |
| 16 | Hibiscus syriacus | Malvaceae | Root bark | 4.6 µM | [99] |
| 17 | Gnaphalium affine | Asteraceae | Whole plants | 2.3-46.1 µM | [100] |
| 18 | Ilex paraguariensis | Aquifoliaceae | Leaves | 1-100 µM | [101] |
| 19 | Belamcanda chinensis | roots | 6.8-27 µM | [102] | |
| 20 | Solanum lycopersicum Mill | Solanaceae | Fruit | 87.2% | [103] |
| 21 | Garcinia latissima Miq. | Clusiaceae | Leaves | 66.42% (at 100 µg/mL) | [55] |
| 22 | Garcinia daedalanthera Pierre | Clusiaceae | Bark | 55.71% (at 100 µg/mL) | [104] |
| 23 | Garcinia xanthochymus | Clusiaceae | Pericarp | 65.17% (at 100 µg/mL) | [105] |
| 24 | Lythrum salicaria L | Lythraceae | Aerial parts | 37.8% (at 10 µg/mL) | [106] |
| 25 | Geum urbanum L | Rosaceae | Root, Rhizome | 30.4% (at 10 µg/mL) | [106] |
| 26 | Rubus idaeus L | Rosaceae | Leaves | 36.1% (at 10 µg/mL) | [106] |
| 27 | Rubus fruticosus L. | Rosaceae | Leaves | 30.7% (at 10 µg/mL) | [106] |
| 28 | Potentilla erecta (L.) Rausch | Rosaceae | Rhizome | 37.4% (at 10 µg/mL) | [106] |
| 29 | Filipendula ulmaria L | Rosaceae | Aerial part | 57.4% (at 10 µg/mL) | [106] |
| 30 | Agrimoria eupatoria L | Rosaceae | Aerial part | 55.2% (at 10 µg/mL) | [106] |
| 31 | Aesculus hippocastanum L | Hippocastanaceae | Bark | 62% (at 10 µg/mL) | [106] |
| 32 | Quercus robur L. | Fagaceae | Bark | 40.1% (at 10 µg/mL) | [106] |
| 33 | Geranium pratense L. | Geraniaceae | Aerial part | 20.9% (at 10 µg/mL) | [106] |
| 34 | Geranium robertianum L | Geraniaceae | Aerial part | 34.7% (at 10 µg/mL) | [106] |
| 35 | Pistacia terebinthus L. | Anacardiaceae | Leaves | 58% (at 50 µg/mL) | [107] |
| 36 | Pistacia lentiscus L. | Anacardiaceae | Leaves | 74% (at 50 µg/mL) | [107] |
| 37 | Hypericum hircunum L | Hypericaceae | Aerial part | 48% (at 50 µg/mL) | [107] |
| 38 | Cytinus hypocistis (L.) | Cytinaceae | Aerial part | 42% (at 50 µg/mL) | [107] |
| 39 | Cistus salviifolius L. | Cistaceae | Aerial part | 51% (at 50 µg/mL) | [107] |
| 40 | Arbutus unedo L. | Ericaceae | Leaves | 56% (at 50 µg/mL) | [107] |
| 41 | Vitellaria paradoxa C.F.Gaertn | Sapotaceae | Leaves | 67% (at 50 µg/mL) | [107] |
| 42 | Vitellaria paradoxa C.F.Gaertn | Sapotaceae | Root bark | 59% (at 50 µg/mL) | [107] |
| 43 | Argania spinosa (L.) Skeels | Sapotaceae | Fruit pulp | 38.41% (at 200 µg/mL) | [108] |
| 44 | Senna garrettiana (Craib.) | Araliaceae | Heartwood | 62.5% (at 25 µg/mL) | [109] |
| 45 | Dalbergia parvifolia Roxb | Leguminosae | Heartwood | 32% (at 25 µg/mL) | [109] |
| 46 | Eutrema japonicum (Miq.) Koidz. | Brassicaceae | Whole plants | 90.2% (at 100 µg/mL) | [110] |
| 47 | Pistacia khinjuk Stocks | Anacardiaceae | Roots | 58% (at 50 µg/mL) | [111] |
So far, several secondary metabolites that have been identified have potential elastase inhibitor activity, such as polyphenols, flavonoids, tannins, and triterpenoids [84,87,88,92,93,95-97,99,110,112-118]. Some active compounds as elastase inhibitors from these compound groups have been isolated and identified, such as agrimoniin, pedunculagin, epigallocatechin gallate, resveratrol, genistein, parthenolide from Tormetillae Rhizoma [119]; Pyracrenic acid, catechin, piceatannol, arjunolic acid, and betulinic acid from the stems of Callistemon lanceolatus [89]; Maslinic acid, betulinic acid, and 2-hydroxynaringenin-7-O-β-D-glucopyranoside from Cornus kousa [120]; Visconata from Dodonaea viscosa [97]; Hydroxyhibiscone A from Hibiscus syriacus [99]; and ellagitannin from Melaleuca styphelioides [96]. Rhusflavone, robustaflavone, amentoflavone, 3-O-ramnoside, 7-O-glycosides, apigenin vitexin, isovitexin, quercetin, myricetin, and luteolin from plants [113].
Polyphenolic substances, including certain flavonoids and their derivatives, mainly present in herbal plants, have been discovered to influence elastase release and its effects on human cells [113,119]. Flavonoid aglycones demonstrate the suppressive functions of elastase. They are also known for their glycoside and methylated, acetylated, and hydroxylated derivatives. Using the structure-activity relationship study described here, it is possible to identify the chemical groups in which elastase is inhibited, as shown in Figure 3.
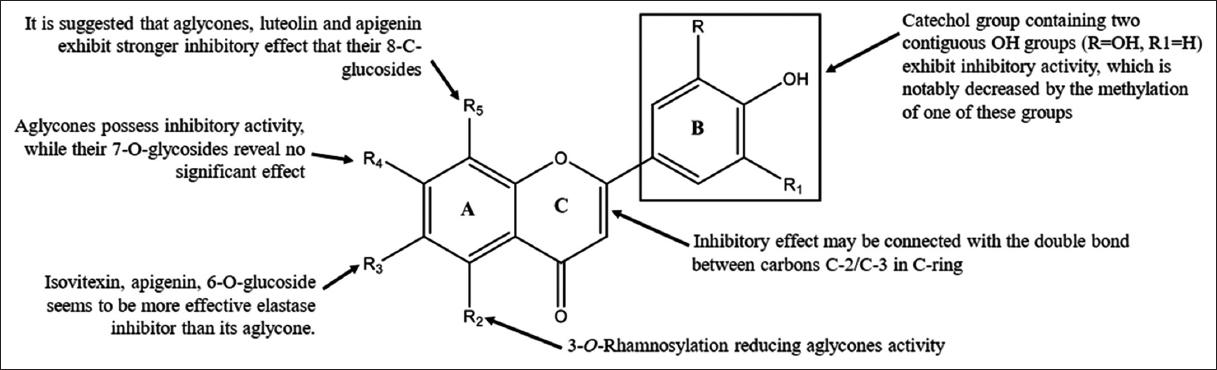 | Figure 3: Structure-Activity Relationship of Chemical group from Flavonoids [113]. [Click here to view] |
Ambarwati et al. (2022) [121] have also carried out molecular docking analysis of some biflavonoid compounds group from Garcinia latissima Miq. to predict the interactions of substances with the elastase enzyme at the molecular level, mainly as inhibitors. These results show that the description of the interaction mechanism is similar to that described by Jakimiuk et al. (2021) [113]. The same study was also reported by (Mechqoq et al., 2022) [108], which showed that the polyphenols and flavonoid compounds groups contained in the extract of Argania spinosa (L.) Skeels) had acted as an elastase inhibitor in vitro and a molecular docking in silico study.
Meanwhile, Wei et al. (2022) [83] conducted a molecular docking analysis study of several phenolic compounds as an antioxidant, elastase, and collagenase inhibition effect, anti-human lung cancer, and anti-SARS-CoV-2 study. Research related to the potential of natural product products as elastase inhibitors has long been proven by Kanashiro et al. (2007) [122], including galangin, kaempferol, quercetin, and myrcetin. This study revealed that the number and position of free hydroxy groups and C2-C3 double bonds in the C ring are essential structural features for the modulating activity of compounds in oxygen-dependent neutrophil function as oxidative bursts [123,124]. Meloni and his colleagues proved that the flavonoid 3-hydroxyfarrerol could act as a reversible and non-competitive inhibitor of NE activity [123]. In addition, a group of secondary metabolite compounds acts as a free radical scavenging and antioxidant inhibitor. Through these two mechanisms, secondary metabolites from natural products have great potential as elastase inhibitors, although they are still limited to in silico, in vitro, and in vivo assays [122,125]. This study also demonstrates that natural phenol compounds are of primary interest and are viewed as a potential replacement for or a way to increase the effectiveness of conventional medications.
4. CONCLUSION AND DIRECTION OF FUTURE
Through oxidative damage and their secondary infection, NETs can destroy viruses and pathogens. In addition, NETs can damage the surrounding tissue due to inflammation. Overactive immune cells can produce NET, which causes lung inflammation. Hence, drugs that can reduce NET formation play a role in reducing the severity of lung disease in COVID-19 patients. One is the therapeutic strategy of inhibiting the oxidative enzymes Nox2 and MPO and the NE enzyme PAD4. In addition, the potential of natural ingredients as inhibitors of the enzyme elastase is very promising to be developed as an alternative to fighting diseases, especially those related to lung infections (especially COVID-19). They were using plants as medicinal raw materials are expected to reduce dependence on synthetic raw materials and vaccines, which have considerable unwanted side effects. The article review results can also be a reference for further researchers to develop and use research to treat various diseases. In addition, it can also be a reference for the government to make policies related to handling a problem in prevention and treatment in cases of the spread of infectious diseases.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
6. FUNDING
This work was supported by research grant of Universitas Negeri Jakarta number 7/PPUI/LPPM/ IV/2022.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All data relating to the manuscript has been provided in script.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2):A global pandemic and treatment strategies. Int J Antimicrob Agents 2020;56:106054. [CrossRef]
2. World Health Organization. COVID-19 Weekly Epidemiology Update. Available from:https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid [Last accessed on 2023 Jan 02].
3. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19):The epidemic and the challenges. Int J Antimicrob Agents 2020;55:105924. [CrossRef]
4. Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. Severe acute respiratory syndrome-related coronavirus:The species and its viruses-a statement of the coronavirus study group. bioRxiv 2020:2020:937862. [CrossRef]
5. Cheng C, Zhang D, Dang D, Geng J, Zhu P, Yuan M, et al. The incubation period of COVID-19:A global meta-analysis of 53 studies and a Chinese observation study of 11 545 patients. Infect Dis Poverty 2021;10:119. [CrossRef]
6. European Centre for Disease Prevention and Control. SARS-CoV-2 Variants of Concern as of 21 December 2022. Available from:https://www.ecdc.europa.eu/en/covid-19/variants-concern [Last accessed on 2023 Jan 02].
7. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19:A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569-78. [CrossRef]
8. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19-final report. N Engl J Med 2020;383:1813-26. [CrossRef]
9. Cordeiro LP, Linhares EO, Nogueira FG, Moreira-Silva D, Medeiros-Lima DJ. Perspectives on glucocorticoid treatment for COVID-19:A systematic review. Pharmacol Rep 2021;73:728-35. [CrossRef]
10. Polverino E, Rosales-Mayor E, Dale GE, Dembowsky K, Torres A. The role of neutrophil elastase inhibitors in lung diseases. Chest 2017;152:249-62. [CrossRef]
11. Heinz A. Elastases and elastokines:Elastin degradation and its significance in health and disease. Crit Rev Biochem Mol Biol 2020;55:252-73. [CrossRef]
12. Fitch PM, Roghanian A, Howie SE, Sallenave JM. Human neutrophil elastase inhibitors in innate and adaptive immunity. Biochem Soc Trans 2006;34:279-82. [CrossRef]
13. Pham CT. Neutrophil serine proteases:Specific regulators of inflammation. Nat Rev Immunol 2006;6:541-50. [CrossRef]
14. Korkmaz B, Moreau T, Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G:Physicochemical properties, activity and physiopathological functions. Biochimie 2008;90:227-42. [CrossRef]
15. Sun Z, Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol 2004;5:182-90. [CrossRef]
16. El Tabaa MM, El Tabaa MM. New putative insights into neprilysin (NEP)-dependent pharmacotherapeutic role of roflumilast in treating COVID-19. Eur J Pharmacol 2020;889:173615. [CrossRef]
17. Guéant JL, Guéant-Rodriguez RM, Fromonot J, Oussalah A, Louis H, Chery C, et al. Elastase and exacerbation of neutrophil innate immunity are involved in multi-visceral manifestations of COVID-19. Allergy 2021;76:1846-58. [CrossRef]
18. Gattinoni L, Gattarello S, Steinberg I, Busana M, Palermo P, Lazzari S, et al. COVID-19 pneumonia:Pathophysiology and management. Eur Respir Rev 2021;30:210138. [CrossRef]
19. Chen R, Lan Z, Ye J, Pang L, Liu Y, Wu W, et al. Cytokine storm:The primary determinant for the pathophysiological evolution of COVID-19 deterioration. Front Immunol 2021;12:589095. [CrossRef]
20. Bhattacharyya R, Iyer P, Phua GC, Lee JH. The interplay between coagulation and inflammation pathways in COVID-19-associated respiratory failure:A narrative review. Pulm Ther 2020;6:215-31. [CrossRef]
21. Korkmaz B, Lesner A, Marchand-Adam S, Moss C, Jenne DE. Lung protection by cathepsin C inhibition:A new hope for COVID-19 and ARDS?J Med Chem 2020;63:13258-65. [CrossRef]
22. Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010;327:291-5. [CrossRef]
23. Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol 2020;20:339-41. [CrossRef]
24. Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol 2012;12:295-305. [CrossRef]
25. Newton AH, Cardani A, Braciale TJ. The host immune response in respiratory virus infection:Balancing virus clearance and immunopathology. Semin Immunopathol 2016;38:471-82. [CrossRef]
26. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [CrossRef]
27. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China:A descriptive study. Lancet 2020;395:507-13. [CrossRef]
28. Tang L, Yin Z, Hu Y, Mei H. Controlling cytokine storm is vital in COVID-19. Front Immunol 2020;11:570993. [CrossRef]
29. Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med 2020;217:e20200678. [CrossRef]
30. Darif D, Hammi I, Kihel A, Saik IE, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis:What goes wrong?Microb Pathog 2021;153:104799. [CrossRef]
31. Nénan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase (MMP-12):A pro-inflammatory mediator?Mem Inst Oswaldo Cruz 2005;100 Suppl 1:167-72. [CrossRef]
32. Cabral-Pacheco GA, Garza-Veloz I, La Rosa C, Ramirez-Acuña JM, Perez-Romero BA, Guerrero-Rodriguez JF, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci 2020;21:9739. [CrossRef]
33. Taddese S, Weiss AS, Neubert RH, Schmelzer CE. Mapping of macrophage elastase cleavage sites in insoluble human skin elastin. Matrix Biol 2008;27:420-8. [CrossRef]
34. Solun B, Shoenfeld Y. Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19. Med Drug Discov 2020;7:100052. [CrossRef]
35. Stevens CS, Oguntuyo KY, Lee B. Proteases and variants:Context matters for SARS-CoV-2 entry assays. Curr Opin Virol 2021;50:49-58. [CrossRef]
36. Voynow JA, Shinbashi M. neutrophil elastase and chronic lung disease. Biomolecules 2021;11:1065. [CrossRef]
37. Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol 2002;451:1-10. [CrossRef]
38. Thierry AR. Anti-protease treatments targeting plasmin(ogen) and neutrophil elastase may be beneficial in fighting COVID-19. Physiol Rev 2020;100:1597-8. [CrossRef]
39. Bai X, Hippensteel J, Leavitt A, Maloney JP, Beckham D, Garcia C, et al. Hypothesis:Alpha-1-antitrypsin is a promising treatment option for COVID-19. Med Hypotheses 2021;146:110394. [CrossRef]
40. Kalyanaraman B. Do free radical NETwork and oxidative stress disparities in African Americans enhance their vulnerability to SARS-CoV-2 infection and COVID-19 severity?Redox Biol 2020;37:101721. [CrossRef]
41. Schönrich G, Raftery MJ. Neutrophil extracellular traps go viral. Front Immunol 2016;7:366. [CrossRef]
42. Violi F, Oliva A, Cangemi R, Ceccarelli C, Pignatelli P, Carnevale R, et al. Nox2 activation in Covid-19. Redox Biol 2020;36:101655. [CrossRef]
43. Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, et al. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol 2018;3:eaar6676. [CrossRef]
44. Kaplan MJ, Radic M. Neutrophil extracellular traps:Double-edged swords of innate immunity. J Immunol 2012;189:2689-95. [CrossRef]
45. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018;18:134-47. [CrossRef]
46. Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol 2012;3:360. [CrossRef]
47. Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 2018;3:eaar6689. [CrossRef]
48. Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med 1978;298:659-68. [CrossRef]
49. Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite:Implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A 1990;87:1620-4. [CrossRef]
50. Debelle L, Tamburro AM. Elastin:Molecular description and function. Int J Biochem Cell Biol 1999;31:261-72. [CrossRef]
51. Baumann L, Bernstein EF, Weiss AS, Bates D, Humphrey S, Silberberg M, et al. Clinical Relevance of elastin in the structure and function of skin. Aesthet Surg J Open Forum 2021;3:ojab019. [CrossRef]
52. Kim MS, Chun KE, Lee DK, Song SH. Evaluation of the efficacy of an elastin-inducing composition containing amino acids, copper, and hyaluronic acid:Results of an open single-center clinical trial study. Cosmetics 2022;9:51. [CrossRef]
53. Wang K, Meng X, Guo Z. Elastin structure, synthesis, regulatory mechanism and relationship with cardiovascular diseases. Front Cell Dev Biol 2021;9:596702. [CrossRef]
54. Mecham RP. Elastin in lung development and disease pathogenesis. Matrix Biol 2018;73:6-20. [CrossRef]
55. Ambarwati NS, Elya B, Desmiaty Y. Anti-elastase activity of methanolic and ethyl acetate extract from Garcinia latissima Miq. J Phys Conf Ser 2019;1402:055079. [CrossRef]
56. Luisetti M, Ma S, Iadarola P, Stone PJ, Viglio S, Casado B, et al. Desmosine as a biomarker of elastin degradation in COPD:Current status and future directions. Eur Respir J 2008;32:1146-57. [CrossRef]
57. Dofferhoff AS, Piscaer I, Schurgers LJ, Visser MPJ, van den Ouweland JM, de Jong PA, et al. Reduced Vitamin K status as a potentially modifiable risk factor of severe coronavirus disease 2019. Clin Infect Dis 2021;73:e4039-46. [CrossRef]
58. Aikawa N, Kawasaki Y. Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther Clin Risk Manag 2014;10:621-9. [CrossRef]
59. Sahebnasagh A, Saghafi F, Safdari M, Khataminia M, Sadremomtaz A, Talaei Z, et al. Neutrophil elastase inhibitor (sivelestat) may be a promising therapeutic option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. J Clin Pharm Ther 2020;45:1515-9. [CrossRef]
60. Tamakuma S, Ogawa M, Aikawa N, Kubota T, Hirasawa H, Ishizaka A, et al. Relationship between neutrophil elastase and acute lung injury in humans. Pulm Pharmacol Ther 2004;17:271-9. [CrossRef]
61. Henriksen PA. The potential of neutrophil elastase inhibitors as anti-inflammatory therapies. Curr Opin Hematol 2014;21:23-8. [CrossRef]
62. Mohamed MM, El-Shimy IA, Hadi MA. Neutrophil elastase inhibitors:A potential prophylactic treatment option for SARS-CoV-2-induced respiratory complications?Crit Care 2020;24:311. [CrossRef]
63. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19:Neutrophil extracellular traps. J Exp Med 2020;217:e20200652. [CrossRef]
64. Tagami T, Tosa R, Omura M, Fukushima H, Kaneko T, Endo T, et al. Effect of a selective neutrophil elastase inhibitor on mortality and ventilator-free days in patients with increased extravascular lung water:A post hoc analysis of the PiCCO Pulmonary Edema Study. J Intensive Care 2014;2:67. [CrossRef]
65. Otsuka R, Miyagi-Shiohira C, Kuwae K, Nishime K, Tamaki Y, Yonaha T, et al. Pancreas preservation with a neutrophil elastase inhibitor, alvelestat, contributes to improvement of porcine islet isolation and transplantation. J Clin Med 2022;11:4290. [CrossRef]
66. Lim CH, Adav SS, Sze SK, Choong YK, Saravanan R, Schmidtchen A. Thrombin and plasmin alter the proteome of neutrophil extracellular traps. Front Immunol 2018;9:1554. [CrossRef]
67. Barrett CD, Bird MA, Moore HB, Moore EE, Yaffe MB. Modeling the effects of human neutrophil elastase on coagulation failure and fibrinolysis. J Am Coll Surg 2018;227:e238. [CrossRef]
68. Gazzaruso C, Paolozzi E, Valenti C, Brocchetta M, Naldani D, Grignani C, et al. Association between antithrombin and mortality in patients with COVID-19. A possible link with obesity. Nutr Metab Cardiovasc Dis 2020;30:1914-9. [CrossRef]
69. Dou Q, Wei X, Zhou K, Yang S, Jia P. Cardiovascular manifestations and mechanisms in patients with COVID-19. Trends Endocrinol Metab 2020;31:893-904. [CrossRef]
70. Parsamanesh N, Pezeshgi A, Hemmati M, Jameshorani M, Saboory E. Neurological manifestations of coronavirus infections:Role of angiotensin-converting enzyme 2 in COVID-19. Int J Neurosci 2022;132:917-24. [CrossRef]
71. John DS, Aschenbach J, Krüger B, Sendler M, Weiss FU, Mayerle J, et al. Deficiency of cathepsin C ameliorates severity of acute pancreatitis by reduction of neutrophil elastase activation and cleavage of E-cadherin. J Biol Chem 2019;294:697-707. [CrossRef]
72. Borges L, Pithon-Curi TC, Curi R, Hatanaka E. COVID-19 and Neutrophils:The relationship between hyperinflammation and neutrophil extracellular traps. Mediators Inflamm 2020;2020:8829674. [CrossRef]
73. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “Cytokine Storm“in COVID-19. J Infect 2020;80:607-13. [CrossRef]
74. Boraldi F, Lofaro FD, Cossarizza A, Quaglino D. The “Elastic Perspective“of SARS-CoV-2 infection and the role of intrinsic and extrinsic factors. Int J Mol Sci 2022;23:1559. [CrossRef]
75. Johansson C, Kirsebom FC. Neutrophils in respiratory viral infections. Mucosal Immunol 2021;14:815-27. [CrossRef]
76. Billett HH, Reyes-Gil M, Szymanski J, Ikemura K, Stahl LR, Lo Y, et al. Anticoagulation in COVID-19:Effect of enoxaparin, heparin, and apixaban on mortality. Thromb Haemost 2020;120:1691-9. [CrossRef]
77. Jin Z, Liu JY, Feng R, Ji L, Jin ZL, Li HB. Drug treatment of coronavirus disease 2019 (COVID-19) in China. Eur J Pharmacol 2020;883:173326. [CrossRef]
78. Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio CuscóM, Ferrández O, Horcajada JP, et al. Azithromycin in the treatment of COVID-19:A review. Expert Rev Anti Infect Ther 2021;19:147-63. [CrossRef]
79. Patel VK, Shirbhate E, Patel P, Veerasamy R, Sharma PC, Rajak H. Corticosteroids for treatment of COVID-19:Effect, evidence, expectation and extent. Beni Suef Univ J Basic Appl Sci 2021;10:78. [CrossRef]
80. Infante M, Ricordi C, Alejandro R, Caprio M, Fabbri A. Hydroxychloroquine in the COVID-19 pandemic era:In pursuit of a rational use for prophylaxis of SARS-CoV-2 infection. Expert Rev Anti Infect Ther 2021;19:5-16. [CrossRef]
81. Bolat E, Eker F, Kaplan M, Duman H, Arslan A, Sarita?S, et al. Lactoferrin for COVID-19 prevention, treatment, and recovery. Front Nutr 2022;9:992733. [CrossRef]
82. Patel TK, Patel PB, Barvaliya M, Saurabh MK, Bhalla HL, Khosla PP. Efficacy and safety of lopinavir-ritonavir in COVID-19:A systematic review of randomized controlled trials. J Infect Public Health 2021;14:740-8. [CrossRef]
83. Wei L, Liao Z, Ma H, Wei J, Peng C. Antioxidant properties, anti- SARS-CoV-2 study, collagenase and elastase inhibition effects, anti-human lung cancer potential of some phenolic compounds. J Indian Chem Soc 2022;99:100416. [CrossRef]
84. Kacem R. Phenolic compounds from medicinal plants as Natural anti-elastase products for the therapy of pulmonary emphysema. J Med Plant Res 2013;7:3499-507.
85. Boudoukha C, Bouriche H, Ortega E, Senator A. Immunomodulatory effects of Santolina chamaecyparissus leaf extracts on human neutrophil functions. Pharm Biol 2016;54:667-73. [CrossRef]
86. Czerwi?ska ME, Kiss AK, Naruszewicz M. Inhibition of human neutrophils NEP activity, CD11b/CD18 expression and elastase release by 3,4-dihydroxyphenylethanol-elenolic acid dialdehyde, oleacein. Food Chem 2014;153:1-8. [CrossRef]
87. Saleem M, Nazir M, Hussain H, Tousif MI, Elsebai MF, Riaz N, et al. Natural phenolics as inhibitors of the human neutrophil elastase (HNE) release:An overview of natural anti-inflammatory discoveries during recent years. Antiinflamm Antiallergy Agents Med Chem 2018;17:70-94. [CrossRef]
88. Radjah SY, Putri KS, Elya B. Elastase inhibitory activity, determination of total polyphenol and determination of total flavonoids, and pharmacognosy study of faloak plant (Sterculia quadrifida R.Br) from east nusa Tenggara-Indonesia. Pharmacogn J 2021;13:758-64. [CrossRef]
89. Kim JH, Byun JC, Kumar A, Bandi R, Hyun CG, Lee NH. Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus. J Med Plants Res 2009;3:914-20.
90. Alasbahi R, Melzig M. The in vitro inhibition of human neutrophil elastase activity by some Yemeni medicinal plants. Sci Pharm 2008;76:471-83. [CrossRef]
91. Kouakou-Siransy G, Sahpaz S, Nguessan GI, DattéJY, Brou JK, Gressier B, et al. Effects of Alchornea cordifolia on elastase and superoxide anion produced by human neutrophils. Pharm Biol 2010;48:128-33. [CrossRef]
92. Wittenauer J, Mäckle S, Sußmann D, Schweiggert-Weisz U, Carle R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015;101:179-87. [CrossRef]
93. Huang Y, Chen L, Feng L, Guo F, Li Y. Characterization of total phenolic constituents from the stems of Spatholobus suberectus using LC-DAD-MSn and their inhibitory effect on human neutrophil elastase activity. Molecules 2013;18:7549-56. [CrossRef]
94. Desmiaty Y, Saputri FC, Hanafi M, Prastiwi R, Elya B. Anti-elastase, anti-tyrosinase, and anti-oxidant of Rubus fraxinifolius stem methanolic extract. Pharmacogn J 2020;12:271-5. [CrossRef]
95. Younis MM, Ayoub IM, Mostafa NM, El Hassab MA, Eldehna WM, Al-Rashood ST, et al. GC/MS profiling, anti-collagenase, anti-elastase, anti-tyrosinase and anti-hyaluronidase activities of a Stenocarpus sinuatus leaves extract. Plants (Basel) 2022;11:918. [CrossRef]
96. Al-Sayed E, Korinek M, Esmat A, Chen GY, Cheng YB, Hsieh PW, et al. Anti-inflammatory, hepatoprotective and antioxidant activity of ellagitannin isolated from Melaleuca styphelioides. Phytochemistry 2020;177:112429. [CrossRef]
97. Uddin Z, Li Z, Song YH, Kim JY, Park KH. Visconata:A rare flavonol having long chain fatty acid from Dodonaea viscosa which inhibits Human neutrophil elastase (HNE). Tetrahedron Lett 2017;58:2507-11. [CrossRef]
98. Tan XF, Kim DW, Song YH, Kim JY, Yuk HJ, Wang Y, et al. Human neutrophil elastase inhibitory potential of flavonoids from Campylotropis hirtella and their kinetics. J Enzyme Inhib Med Chem 2016;31:16-22. [CrossRef]
99. Ryoo IJ, Yun BS, Lee IK, Kim YH, Lee IS, Ahn JS, et al. Hydoroxyhibiscone A, a novel human neutrophil elastase inhibitor from Hibiscus syriacus. J Microbiol Biotechnol 2010;20:1189-91. [CrossRef]
100. Ryu HW, Kim KO, Yuk HJ, Kwon OK, Kim JH, Kim DY, et al. The constituent, anti-inflammation, and human neutrophil elastase inhibitory activity of Gnaphalium affine. J Funct Foods 2016;27:674-84. [CrossRef]
101. Xu GH, Kim YH, Choo SJ, Ryoo IJ, Yoo JK, Ahn JS, et al. Chemical constituents from the leaves of Ilex paraguariensis inhibit human neutrophil elastase. Arch Pharm Res 2009;32:1215-20. [CrossRef]
102. Kim JH, Ban YJ, Baiseitova A, Nyiramana MM, Kang SS, Kang D, et al. Iridal-type triterpenoids displaying human neutrophil elastase inhibition and anti-inflammatory effects from Belamcanda chinensis. Molecules 2021;26:6602. [CrossRef]
103. Ambarwati NS, Armandari MO, Widayat W, Desmiaty Y, Elya B, Arifianti AE, et al. In vitro studies on the cytotoxicity, elastase, and tyrosinase inhibitory activities of tomato (Solanum lycopersicum Mill.) extract. J Adv Pharm Technol Res 2022;13:182-6.
104. Ambarwati NS, Elya B, Desmiaty Y, Omar H. Anti-elastase of leaves and stem bark extract of Garcinia daedalanthera pierre. Int J Pharm Res 2020;12:592-6. [CrossRef]
105. Desmiaty Y, Ambarwati NS, Elya B, Atmanto D, Ahmad I. Elastase inhibitory activity of methanol extract and n-hexane extract of Garcinia xanthochymus Pericarp. J Phys Conf Ser 2021;1869:012063. [CrossRef]
106. Piwowarski JP, Kiss AK, Koz?owska-Wojciechowska M. Anti-hyaluronidase and anti-elastase activity screening of tannin-rich plant materials used in traditional Polish medicine for external treatment of diseases with inflammatory background. J Ethnopharmacol 2011;137:937-41. [CrossRef]
107. Chiocchio I, Mandrone M, Sanna C, Maxia A, Tacchini M, Poli F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind Crops Prod 2018;122:498-505. [CrossRef]
108. Mechqoq H, Hourfane S, El Yaagoubi M, El Hamdaoui A, da Silva Almeida JRG, Rocha JM, et al. Molecular docking, tyrosinase, collagenase, and elastase inhibition activities of argan by-products. Cosmetics 2022;9:10024. [CrossRef]
109. Sakunpak A, Saingam W. Screening and compound isolation from selected Thai herbal medicine for anti-hyaluronidase and anti-elastase activities. Proc RSU Int Res Conf 2020:305-11.
110. Szewczyk K, Pietrzak W, Klimek K, Miazga-Karska M, Firlej A, Flisi?ski M, et al. Flavonoid and phenolic acids content and in vitro study of the potential anti-aging properties of Eutrema japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland. Int J Mol Sci 2021;22:6219. [CrossRef]
111. Tilkat EA, Batibay H, Yener I, Yilmaz PK, Akdeniz M, Kaplan A, et al. Determination of enzyme inhibition potential and anticancer effects of Pistacia khinjuk stocks raised in in vitro and in vivo conditions. Agronomy 2021;1:154. [CrossRef]
112. Eun CH, Kang MS, Kim IJ. Elastase/collagenase inhibition compositions of Citrus unshiu and its association with phenolic content and anti-oxidant activity. Appl Sci 2020;10:10144838. [CrossRef]
113. Jakimiuk K, Gesek J, Atanasov AG, Tomczyk M. Flavonoids as inhibitors of human neutrophil elastase. J Enzyme Inhib Med Chem 2021;36:1016-28. [CrossRef]
114. Yücel Ç, ?eker Karatoprak G, Yalç?nta?S, BöncüTE. Ethosomal (-)-epigallocatechin-3-gallate as a novel approach to enhance antioxidant, anti-collagenase and anti-elastase effects. Beilstein J Nanotechnol 2022;13:491-502. [CrossRef]
115. Feng L, Liu X, Zhu W, Guo F, Wu Y, Wang R, et al. Inhibition of human neutrophil elastase by Pentacyclic triterpenes. PLoS One 2013;8:e82794. [CrossRef]
116. Lee KK, Cho JJ, Park EJ, Choi JD. Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechu as a new anti-ageing agent. Int J Cosmet Sci 2001;23:341-6. [CrossRef]
117. Kabeya LM, Fuzissaki CN, Andrade MF, Azzolini AE, Taleb-Contini SH, Vermelho RB, et al. 4-methylcoumarin derivatives inhibit human neutrophil oxidative metabolism and elastase activity. J Med Food 2013;16:692-700. [CrossRef]
118. Rungruang R, Panichakul T, Rattanathavorn W, Kaisangsri N, Kerdchoechuen O, Laohakunjit N, et al. Effects of extraction methods on the flavonoid and phenolic contents and anti-aging properties of Rhyncholaeliocattleya Haw Yuan Beauty extracts. ScienceAsia 2021;47:698-706. [CrossRef]
119. Hrenn A, Steinbercher T, Labahn A, Schwager J, Schempp CM, Merfort I. Plant phenolic inhibit neutrophil elastase. Planta Med 2006;72:1127-31. [CrossRef]
120. Sultana N, Lee NH. Antielastase and free radical scavenging activities of compounds from the stems of Cornus kousa. Phytother Res 2007;21:1171-6. [CrossRef]
121. Ambarwati NS, Azminah A, Ahmad I. Molecular docking, physicochemical and drug-likeness properties of isolated compounds from Garcinia latissima Miq. on elastase enzyme:In silico analysis. Pharmacogn J 2022;14:282-8. [CrossRef]
122. Kanashiro A, Souza JG, Kabeya LM, Azzolini AE, Lucisano-Valim YM. Elastase release by stimulated neutrophils inhibited by flavonoids:Importance of the catechol group. Z Naturforsch C J Biosci 2007;62:357-61. [CrossRef]
123. Meloni F, Ballabio P, Gorrini M, De Amici M, Marena C, Malandrino S, et al. Effects of 3'-hydroxyfarrerol (IdB 1031), a novel flavonoid agent, on phagocyte products. Inflammation 1995;19:689-99. [CrossRef]
124. Siedle B, Hrenn A, Merfort I. Natural compounds as inhibitors of human neutrophil elastase. Planta Med 2007;73:401-20. [CrossRef]
125. Kanashiro A, Kabeya LM, Polizello AC, Lopes NP, Lopes JL, Lucisano-Valim YM. Inhibitory activity of flavonoids from Lychnophora sp. on generation of reactive oxygen species by neutrophils upon stimulation by immune complexes. Phytother Res 2004;18:61-5. [CrossRef]