1. INTRODUCTION
Wheat Triticum aestivum L. is a major grain crop grown worldwide within the family Poaceae [1]. It is thought this plant has originated from southeastern parts of Turkey 10,000 years BC [2]. Whereas in Iraq, bread wheat has been domesticated 9500 years BC [3]. Wheat is grown in Iraq in winter season. Based on 2020 statistics, the estimated Iraqi production and growing area of wheat were 6,238,392 tons and 2,143,421 ha, respectively [4]. About 46 different wheat varieties have been reported to be grown in Iraq [5]. Wheat is attacked by several pests including nematodes [1]. At least, four nematode groups infect wheat including cereal cyst, root knot, root lesion, and seed gall nematodes [5]. Anguina tritici (Steinbuch, 1799) Chitwood, 1935, or ear cockle nematode is a major plant pathogenic nematode infecting wheat causing serious losses worldwide [6]. It has been described for the 1st time by Needham since 1743 [1]. Seed gall disease has been reported in Iraq for the 1st time since 1921 [7] but the causal agent A. tritici was recently confirmed by molecular approach [8]. This disease can cause yield losses ranged 10.76–75% in wheat [9,10]. Limited studies conducted in Iraq were investigated A. tritici control which were based on cultural, chemical, and biological methods [11-13]. Due to the limited molecular studies regarding seed gall disease in Iraq, this study, therefore, was initiated to identify the nematode causing seed gall disease in Wasit and Kirkuk provinces and its phylogenetic relatedness and to investigate the efficacy of the bioagent Trichoderma harzianum against seed gall disease on three wheat in the study area.
2. MATERIALS AND METHODS
2.1. Pathogenic Nematode Isolation and Inoculum Preparation
In 2021 growing season, gall samples were obtained from wheat fields located at Kirkuk and Waset provinces in Iraq. Ten galls were socked in 500 ml sterilized distilled water for 24 h at ambient temperature to release the second stage juveniles (J2). The number of J2 nematode was estimated using microscopic examination at 40X [14].
2.2. Molecular Conformation of the Pathogenic Nematode
Two seed galls from each location were crushed with liquid nitrogen and DNA extraction was performed using commercial DNA extraction kit (Bioneer, South Korea), following the manufacturer’s instructions. PCR amplification was performed following [15], using AccuPower PCR PreMix commercial kit (Bioneer, South Korea) and ITS rRNA universal primers (rDNA2: TTGATTACGTCCCTGCCCTTT and rDNA1: ACGAGCCGAGTGATCCACCG) [16,17]. DNA fragment amplified was visualized by ethidium bromide agarose gel electrophoresis [18]. PCR products were directly sequenced (Macrogen, South Korea). Sequences were analyzed using MEGA11 [19] and Sequence Demarcation Tool Version 1.2 (SDT v 1.2) [20] software packages.
2.3. Field Experiment
Local wheat varieties T. aestivum L. vars. Cham 6, Baraka, and Aras were tested against seed gall nematode infection. The experimental field was plotted into 1 m2 areas with three replicates of each treatment. T. harzianum suspension (in the form of trichozone biopesticide, Al-Joud Company for Industry and Modern Agriculture, Iraq), was applied by foliar spraying or root watering at concentration 2 g/L. Nematode inoculum, adjusted to 10,000 J2 individuals’ concentration, was applied at 1000 mL/m2 rate, 25 days of seed sowing. All agricultural practices necessary for plant growth were followed, during the experiment. Infectivity percent and disease reduction (DR) were calculated using the following equation:
 |
Microsoft Excel 2010 was used to analyze data calculated.
3. RESULTS AND DISCUSSION
Sequence comparison confirmed DNA fragments amplified [Figure 1] were identical to18S small subunit (SSU) ribosomal DNA genomic region of A. tritici retrieved from the NCBI [Figure 2]. A. tritici isolated from Wasit and Kirkuk scored 100% maximum nucleotide (nt) sequence identities to equivalent GenBank sequences from Mexico (AF363107) and India (JF826516), respectively, suggesting their common origin [Figure 2]. Neighbor-joining (NJ) phylogenic tree, based on SSU nt sequences, confirmed the relatedness when grouped Wasit and Kirkuk sequences to relevant isolates from Mexico and India [Figure 2]. Despite the high identity, Wasit and Kirkuk isolates showed that they could be two variants when N-J phylogenetic tree separated them into two clades within A. tritici branch [Figure 3]. Thus, SSU could be useful molecular loci for identification, differentiation, and phylogeny reconstruction of A. tritici in Iraq [8,21].
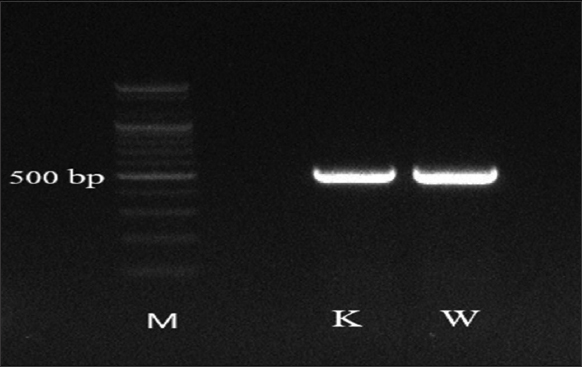 | Figure 1: Ethidium bromide stained gel pattern shows ~ 500 bp amplified by rDNA2/rDNA1 primer set from seed galls collected. K: Kirkuk sample, W: Wasit sample, and M: 100 bp DNA marker (Bioneer, S. Korea). [Click here to view] |
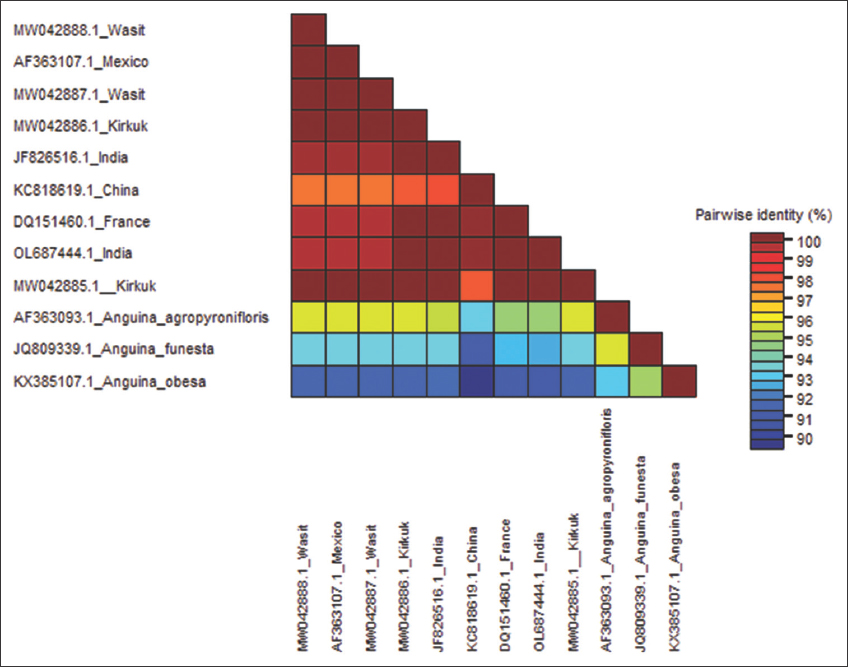 | Figure 2: Three color mode matrixes showing A. tritici identities, constructed from partial 18S SSU ribosomal DNA sequences of Kirkuk and Wasit and equivalent sequences from the GenBank. A. agropyronifloris Norton, 1965, A. funesta Price, Fisher and Kerr, 1979, and A. obesa Mobasseri, Pedram, Pourjam and Bertozzi, 2017, were used as out-group comparisons. Data were analyzed using Muscle algorithm. This matrix was generated using Sequence Demarcation Tool Version 1.2 (SDTv1.2) software package [20]. [Click here to view] |
 | Figure 3: Neighbor-joining phylogenetic tree constructed from partial 18S SSU rDNA nucleotide sequences of Anguina tritici from Iraq (marked with ?) and equivalent sequences from the GenBank. A. agropyronifloris Norton, 1965, A. funesta Price, Fisher and Kerr, 1979, and A. obesa Mobasseri, Pedram, Pourjam and Bertozzi, 2017, were used as out-group comparisons. Data were analyzed using Maximum Composite Likelihood method. This tree was constructed by MEGA 11 software [19]. [Click here to view] |
This variation may be related to the selective pressure of many constraints favoring certain pathotypes over others in wheat growing area including varieties grown, alternative hosts available, and limited movements of nematode-infected plant materials, nationwide [22,23].
Field experiment revealed that T. harzianum treatments could decrease infectivity percent and reduce seed gall disease in all three varieties compared to J2 only treatment. Root watering treatment showed high efficiency to control seed gall disease scoring 15.4% lowest infectivity [Figure 4] and 46.71% highest DR [Figure 5] in Aras variety, compared to foliar spraying. Root watering treatment with T. harzianum might minimize A. tritici infection much effectively than foliar spraying through direct interaction with J2 individuals, systemic induced resistance, and/or promoting or inducing plant growth [24,25]. This study confirmed the incidence of A. tritici in two Iraqi provinces, Kirkuk and Wasit. Based on phylogenetic relatedness and high identity percentage, these two geographical variants may have been introduced into Iraq through imported contaminated wheat seeds in the near past [26]. Except Saber-Beg, all wheat varieties are seed gall sensitive [27-29]. Aras, Baraka, and Cham6 wheat varieties showed to be sensitive to seed gall disease infection. Field experiment revealed that Aras wheat variety was less susceptible to seed call infection when scored 28.9% infectivity percent followed by Baraka and Cham 6 which scored 29.6 and 33.1% infectivity, respectively [Figure 4]. These three varieties are cultivated in Iraq due to their high yield quality and quantity compared to Saber-Beg. Bioagent treatment of desirable varieties can be the most efficient among others controlling methods as it offers a sustainable, eco-friendly alternative nematicide, to manage seed gall disease in Iraq [30]. Besides, it can enhance wheat production through promoting plant growth [24,25].
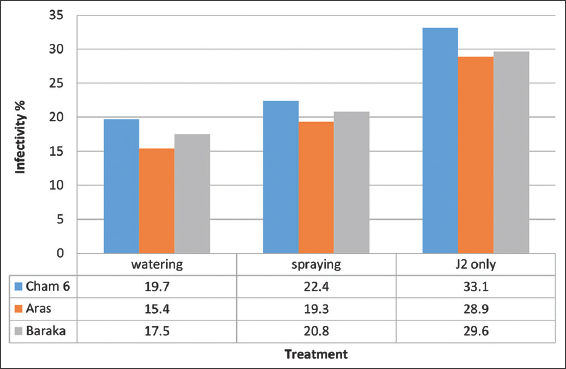 | Figure 4: T. harzianum field treatments of three wheat varieties (Cham 6, Aras, and Baraka) against A. tritici using foliar spraying and root watering applications. Root watering treatments show the lowest infectivity percentages. J2: Represents untreated infarcted control. This bar chart was generated using Microsoft Excel 2010. [Click here to view] |
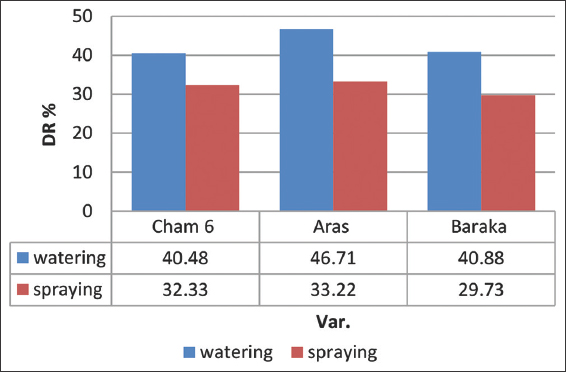 | Figure 5: Disease reduction (DR) percentages resulted from T. harzianum field treatments of three wheat varieties (Cham 6, Aras, and Baraka) against A. tritici using foliar spraying and root watering applications. Root watering treatments show the lowest infectivity percentages. J2 only: Untreated infarcted control. This bar chart was generated using Microsoft Excel 2010. [Click here to view] |
4. CONCLUSIONS
This study confirmed the detection of A. tritici collected from two Iraqi provinces, Kirkuk and Wasit. Phylogenetic relatedness showed high identity percentage of the Iraqi seed gall nematode to the equivalent GenBank isolates from Mexico and India. Root treatment with T. harzianum was much effective than foliar treatment, against A. tritici infection, when decrease the infectivity percent of seed galls in all treatments. T. aestivum L. var. Aras was less susceptible to seed gall infection compared to Baraka and Cham 6 varieties. Bioagent treatment of desirable varieties can be the most efficient among others controlling methods as it offers a sustainable, eco-friendly alternative nematicide, to manage seed gall disease in Iraq.
5. ACKNOWLEDGMENT
Authors would like to thank the staff of the Seed Inspection and Certification directorate/Ministry of Agriculture and Miss Noor Raad Khuder and Mr. Ali Majid Jawad (Almusaib Bridge for Scientific and Lab Equipment) for their technical assistance.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors requirements/guidelines.
7. FUNDING
There is no funding to report.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
The authors confirmed that all the relevant data were included in the article.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Skantar AM. Anguina tritici (Wheat Seed Gall Nematode). Invasive Species Compendium. Wallingford, UK:CABI;2018. Available from:https://www.cabi.org/isc/datasheet/5388 [Last accessed on 2022 Apr 03].
2. Shewry PR. Wheat. J Exp Bot 2019;60:1537-53. [CrossRef]
3. Hirst KK. Wheat Domestication. Thought Co.;2021. Available from:https://www.thoughtco.com/wheat-domestication-the-history-170669 [Last accessed on 2022 May 16].
4. FAOSTAT, Crops and Livestock Products. Available from:https://www.fao.org/faostat/en/#data/QCL [Last accessed on 2022 May 16].
5. Wheat Atlas. Available from:http://wheatatlas.org [Last accessed on 2022 May 16].
6. Dababat AA, Muminjanov H, Smiley R. Nematodes of small grain cereals current status and research. In:5th International Cereal Nematode Initiative Workshop, 12–15 September 2015. Ankara, Turkey:Food and Agriculture Organization of the United Nations, Ankara, Turkey, 2015.
7. Rao RS. A Preliminary List of Insect Pest of Iraq. Bombay:Times Press;1921.
8. Ami SN, Taher IE, Hussen FS, Ahmed AI. First molecular identification of wheat seed gall nematode Anguina tritici races parasitized on wheat in Iraq. Acta Univ Sapientiae Agric Environ 2019;11:5-15. [CrossRef]
9. Stephan ZA, Antoon BG. Biotypes of ear cockle nematode Anguina tritici in Iraq. Curr Nematol 1990;1:85-8.
10. Ami SN, Taher IE. Survey, races identification and host range of wheat seed gall nematode Anguina tritici Duhok Province, Kurdistan region Iraq. J Agric Vet Sci 2014;7:44-8. [CrossRef]
11. Ami SN, Mustafa SA. Susceptibility of certain wheat and barley cultivars to seed gall nematode Anguina tritici (Steinbech,1979) Filipjev, 1936. Mesopotamia J Agric 2012;40:217-23. [CrossRef]
12. Hassan AA, Hindi YK. Integrated control of Anguina tritici by some nematodacides and Trichoderma harzianum isolated from wheat fields in Salah Aldin governorate. Tikrit J Agric Sci 2015;15:85-98.
13. Al-Taie AH, Al-Zubaidi NK. Interaction efficiency of Trichoderma spp. and some plant extracts against ear-cockle disease. J Appl Biol Biotechnol 2022;10:102-7.
14. Fattah FA, Al-Assas K. Histopathological comparison of galls induced by Anguina tritici with galls subsequently colonised by Rathayibacter tritici in wheat. Nematol Mediterr 2010;109:195-8.
15. Szalanski AL, Sui DD, Harris TS, Powers TO. Identification of cyst nematodes of agronomic and regulatory concern by PCR-RFLP of ITS1. J Nematol 1997;29:255-67.
16. Cherry T, Szalanski AL, Todd TC, Powers TO. The internal transcribed spacer region of Belonolaimus (Nemata:Belonolaimidae). J Nematol 1997;29:23-9.
17. Vrain TC, Wakarchuk AC, Levesque AC, Hamilton RI. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam Appl Nematol 1992;15:563-73.
18. Sambrook JF, Russell D. Condensed Protocols:From Molecular Cloning:A Laboratory Manual. New York:Cold Spring Harbor Laboratory Press;2006. [CrossRef]
19. Tamura K, Stecher G, Kumar S. MEGA11:Molecular evolutionary genetics analysis version 11. Mol Biol Evol 2021;38:3022-7. [CrossRef]
20. Muhire BM, Varsani A, Martin DP. SDT:A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS One 2014;9:e108277. [CrossRef]
21. Carta LK, Li S. Improved 18S small subunit rDNA primers for problematic nematode amplification. J Nematol 2018;50:533-42. [CrossRef]
22. Lambert K, Bekal S. Introduction to plant-parasitic nematodes. Plant Health Instr 2002;10:1094-1218. [CrossRef]
23. Montarry J, Mimee B, Danchin EG, Koutsovoulos GD, Ste-Croix DT, Grenier E. Recent advances in population genomics of plant-parasitic nematodes. Phytopathology 2021;111:40-8. [CrossRef]
24. Poveda J, Abril-Urias P, Escobar C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance:Trichoderma, mycorrhizal and endophytic Fungi. Front Microbiol 2020;11:992. [CrossRef]
25. TariqJaveed M, Farooq T, Al-Hazmi AS, Hussain MD, Rehman AU. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. J Invertebr Pathol 2021;183:107626. [CrossRef]
26. Mackesy DZ, Sullivan M. CPHST Pest Datasheet for Anguina tritici. USDA-APHISPPQ-CPHST;2016.
27. AL-Jobouri M, AL-Jobouri A, AL.Qaissi E. Susceptibility of bread wheat cultivars to wheat gall nematode and a study some of the gene action of infection percentage. Kirkuk Univ J Sci Stud 2010;5:112-21. [CrossRef]
28. Qassem NE, AL-Taae HH, Thanoon AH. Screening of some varieties of wheat for infestation by the seed gall nematode Anguina tritici. Plant Cell Biotechnol Mol Biol 2021;22:94-105.
29. AL-Jobouri J, AL-Jobouri R, Alsaedy H. Varietal and biological resistance for nematode no des of wheat Anguina tritici in genotypes of bread wheat Triticum aestivum L. Kirkuk Univ J Sci Stud 2021;11:96-111.
30. B?aszczyk L, Siwulski M, Sobieralski K, Lisiecka J, J?dryczka M. Trichoderma spp. application and prospects for use in organic farming and industry. J Plant Prot Res 2014;54:309-17. [CrossRef]