1. INTRODUCTION
Macrofungi are basidiomycetous and ascomycetous fungi, which are naturally found growing on lignin-cellulosic substrates in the forest and grassland and even in agricultural areas. They are considered nontimber forest inhabitants that play important ecological roles as decomposers of massive forest litters and organic matter to maintain soil fertility and act as an indispensable partner of many species of trees through mycorrhizae. Traditionally and seasonally, wild macrofungi have been consumed by humans due to their nutrient compositions and therapeutic values. They are valuable sources of proteins, fibers, carbohydrates, functional lipids, vitamins, minerals, and mycochemicals responsible for several biological activities such as antioxidants, antibacterial, antidiabetic, antihypertension, anticoagulant, and anti-inflammation [1–4]. Thus, it is necessary to explore more macrofungi, particularly those unrecorded species with promising cultivation and nutraceutical potentials.
To date, most studies on the ethnomycology, collection, identification, and species listing of naturally occurring macrofungi in the Philippines have been conducted in different areas of Luzon Island including six Aeta tribal communities in Pampanga, Zambales, Tarlac [5], Aeta community in Mt. Nagpale, Abucay, Bataan [6], Kalanguya communities of Carranglan, Nueva Ecija [7], Gaddang communities in Nueva Vizcaya [8], Bazal-Baubo Watershed, Aurora [9], Mt. Bangcay, Cuyapo, Nueva Ecija [10], Central Luzon State University Campus, Science City of Munoz, Nueva Ecija [11], Mt. Mingan, Gabaldon, Nueva Ecija [12], Angat Watershed Reservation, Bulacan [13], La Union Province, Northern Luzon [14], Mt. Makiling Forest Reserve, Los Banos, Laguna [15], Mt. Maculot, Cuenca, Batangas [16], Mt Palay-Palay/Mataas na Gulod Protected Landscape, Southern Luzon [17], Taal Volcano Protected Landscape, Southern Luzon [18], and Mt. Malinao, Albay [19]. Moreover, Biadnes and Tangonan [20] reported different species of Basidiomycetes found in Mt. Apo in Mindanao. Literature shows that no work has been documented on the distribution and species listing of wild mycoflora of Sitio Canding in Tarlac Province and hence this study.
Sitio Canding is one of the sitios of Barangay Maasin, Municipality of San Clemente, Province of Tarlac in Central Luzon. The sitio is situated between 15° 38′ N latitude and 120° 18′ E longitude with a mean elevation of 233.4 m above the sea level. It consists of hills, mountain, narrow valleys, waterfall, and river. The Canding Falls and their downstream river provide a continuous supply of water to man-made secondary forest and agroecosystem, thereby considered as one of the famous ecotourism sites in Tarlac. With the notable disturbance brought by tourists and land conversion by the local residence in the area, there is possibility that some of the native species particularly macrofungal resources become extinct. Therefore, prior to extinction, it is indeed imperative to investigate the existence of macrofungal species and rescue their cell lines for possible conservation and utilization.
 | Figure 1: Google earth map of Tarlac Province showing the location of the Sitio Canding, Brgy. Maasin, Municipality of San Clemente. [Click here to view] |
This study was conducted to document and profile the growth habit and natural habitat (humidity, temperature, substrate type, and pH of substrate) and collect and morphologically identify naturally occurring macrofungi in Sitio Canding. Moreover, the occurrence of macrofungi in three collection sites, in three consecutive collection months, and in five elevation ranges was also determined. A taxonomic list of macrofungal species was established. The mycelia of collected mushrooms were rescued using tissue culture technique for macrofungi.
2. MATERIALS AND METHODS
2.1. Study Site
The secondary forest and agroecosystem along Canding River in Sitio Canding, Barangay Maasin, Municipality of San Clemente, Province of Tarlac in Central Luzon, Philippines (Fig. 1), are the sites of the study. Ocular inspection of the study site was done. The vegetation and accessibility of the area were considered in selecting the sampling sites.
2.2. Field Sampling of Macrofungi
Purposive and opportunistic sampling was used in this study. Documentation of macrofungi and their natural habitat and collection were done in three transect lines, each with 1,000 m long (representing collection site). The study site was visited once a month from August to October 2019. Relative humidity, temperature, rainfall, substrate type, and growth habit of macrofungi were recorded. The location and elevation of the habitat of each macrofungal species were determined using a Global Positioning System (GPS). Macrofungi and their substrates were carefully collected and individually placed in properly labeled brown paper bag and were brought to the laboratory for morphological identification and pH of substrate determination. Samples were also tissue-cultured in potato dextrose agar to rescue the mycelia.
2.3. Morphological Identification
The collected macrofungi were subjected to the thorough analysis of macro- and microscopic attributes as a basis of morphological identification. The identity of macrofungi was compared to the species listings and taxonomic works of Arenas et al. [16,17], De Leon et al. [5,7], Tadiosa et al. [9], Quimio [21], and Peterson’s Field Guide on Mushrooms of McKnight and McKnight [22] and was authenticated by a mycologist.
2.4. Data Analysis
The percentage composition of the macrofungal taxa was computed to determine the occurrence of macrofungi in three collection sites, in three collection months, and in five elevation ranges.
3. RESULTS AND DISCUSSION
3.1. Natural Habitat of Macrofungi
Sitio Canding is one of the hilly and mountainous areas in Tarlac with narrow valleys, waterfalls, river, secondary forest and agroforest with moderate to vast vegetation, and agricultural lands. Three collection sites were identified and sampling was done once a month from August to October 2019. Collection site A is situated along a trail passing through a secondary forest with old trees, while collection site B is situated along a river passing through multistorey agroforestry systems. On the other hand, collection site C is situated along a river passing through agroforest and agricultural lands. During the 3 months of sampling in the three collection sites, a total of 116 macrofungi were documented and collected. The growth habits and natural habitats (including temperature and humidity) of the different macrofungi were recorded. The growth habit of most macrofungi was solitary, followed by solitary to gregarious, and only few were resupinate. Most of the collected specimens were classified as wood-rotting macrofungi, since they were found favorably growing on dead roots, trunk, and branches of trees, fallen logs, decaying twigs, rotten stumps, and bamboo as their natural substrates. Some were observed on soil, pile of decaying leaf litters, termite mound, and corncob. The mentioned substrates had a pH range of 4.1–7.1. However, the majority of the macrofungi recorded a slightly acidic substrate. The existence of these macrofungi on their substrate could be either saprophytic, parasitic, or symbiotic, which is necessary to investigate in further studies. Indeed, macrofungi play a very crucial role in the decomposition process of forest litters as their substrates, thereby contributing enormously to the nutrient cycling of the soil.
Temperature and humidity (atmospheric moisture) are the two most important environmental factors that significantly influenced macrofungal growth. In this study, temperature and humidity were measured between 7:00 and 10:00 am in the natural habitat of individual mushroom. The temperature varied in the three collection sites and in three collection months. The temperature ranged from 25°C to 28.5°C. The change of temperature in the three collection sites was due to the varying elevation. As elevation increased, the temperature decreased. However, humidity varied only in three collection months. The humidity during the first 2 months was 92%, whereas the last month was noted at 80%. This variability of humidity and temperature in the three collection months could be accounted to the precipitation or rainfall. Among the three collection months, the highest rainfall was recorded in August, followed by September. On the other hand, October had the lowest precipitation. This could be the main reason for higher temperature, lower humidity, and very few macrofungi collected during this month. The present study also showed that rainfall of 300 mm and above could stimulate emergence of fruiting bodies of many macrofungal species as observed during August and September. This could be explained by the sufficient moisture absorbed by the spores, which activates the germination process, and then the formation of hypha and mycelia and eventually lead to the development of fruiting bodies. Hence, the amount of rainfall could be very useful indicator of the abundance of growing macrofungi in their natural habitat. This supports the belief of Filipinos particularly mushroom hunters and growers that mushrooms are abundantly growing after the heavy rain. However, Kauserud et al. [23] reported that the emergence of many species of macrofungi occurs after rainfall within 10, 15, or 30 days. Therefore, rainfall provides favorable temperature and humidity that allows the proliferation of naturally occurring macrofungi and has a great effect on macrofungal distribution. The profile of natural habitat of macrofungi obtained in the present study is very important in generating practical technologies for the production, conservation, and utilization of these wild macrofungi.
3.2. Physical Distribution of Macrofungi
Given the profile of natural habitat and climatic conditions, the physical distribution of macrofungi in three collection sites, three collection months, and five elevation ranges was investigated (Table 1). Among the three collection sites, collection site A recorded the highest percentage composition of macrofungal species (59.72%), followed by collection site B (50.00%). On the other hand, collection site C had the lowest percentage composition (13.89%). The distribution of 116 macrofungi in the three collection sites based on the GPS coordinates is shown in Figure 2. Higher composition of macrofungi species in collection sites A and B could be attributed to the availability of massive forest litters as substrates for macrofungi to grow, since the two sites are still consisting of secondary forest with old trees and multistorey agroforestry systems, respectively. However, the poor macrofungal composition in collection site C could be due to the disturbed habitat as a result of intensive farming activities by the residents. Therefore, the availability of lignin-cellulosic substrates and undisturbed habitat is positively correlated with the macrofungal species composition in a given area.
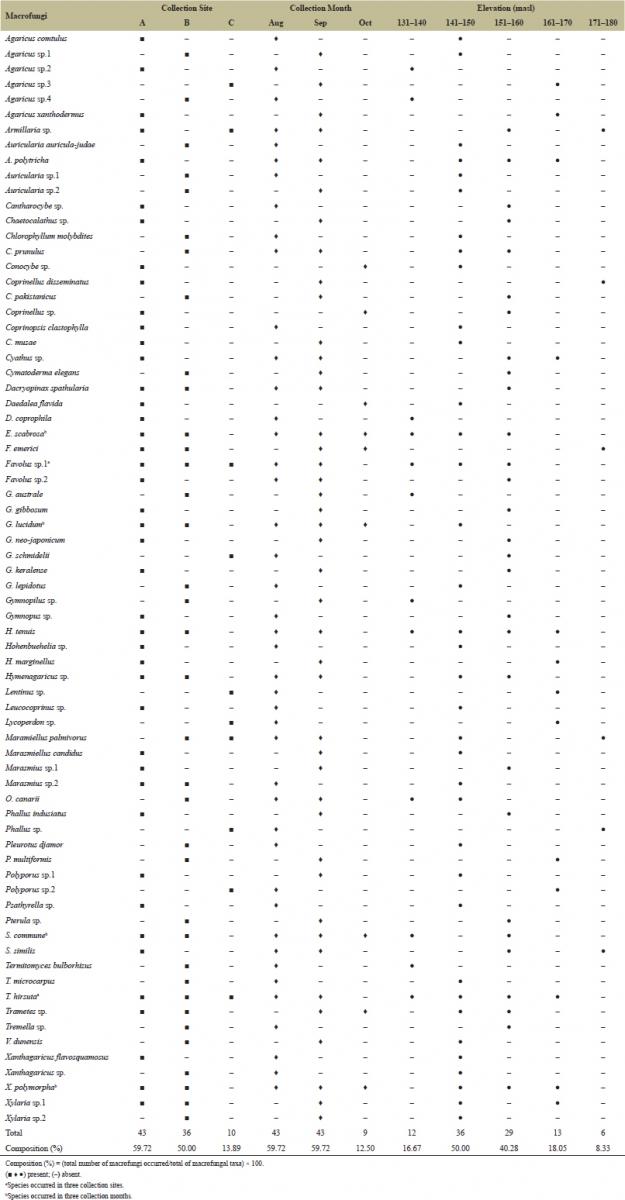 | Table 1. Distribution of 116 collected macrofungi in the three collection sites, three collection months, and five elevation ranges. [Click here to view] |
In three collection months, both August and September registered the highest percentage composition of macrofungal species (59.72%), whereas October had the lowest (12.50%). The distribution of macrofungi in three collection months can be correlated with the changing climatic conditions discussed in the preceding section. The low rainfall that results in a significant increase in temperature to 28.5°C and downshift of humidity to 80% inhibited the growth of many macrofungi in October. The successful growth of some macrofungi despite the quite unfavorable conditions could be explained by their strong adaptability mechanism and by the presence of massive substrate in their natural habitat. Chang and Miles [24] reported that the optimal temperature for the development of fruiting bodies can range from 10°C to 18°C. However, to our knowledge, the optimal temperature for fruiting body development may vary depending on the strain and species of mushroom. In fact, mushrooms can be classified as temperate, semitemperate, and tropical species based on their optimum temperature [25]. On the other hand, fruiting body formation of macrofungi usually requires relatively high humidity [26]. In the taxonomic study of macrofungi in Mt Maculot, Cuenca, Batangas, the highest macrofungal composition was documented in December (63%), followed by February (59%), June (58%), and September (54%) [16]. Moreover, Ramel [27] reported that, among the three collection months, January recorded the highest macrofungal composition of 65.79% in Barobbob Watershed, Bayombong, Nueva Vizcaya. These studies, however, contradict the present results that August and September showed the highest macrofungal composition. This could be explained by the fact that different regions in the country have different rainfall distributions. The Philippines has four types of climate depending on the distribution of rainfall and the period of the dry season. The Province of Tarlac is under climate type 1, wherein the maximum rainfall period is from June to September and dry period is from November to April. Therefore, macrofungal distribution may vary depending on the climatic conditions at the time of collection and of the specific regions. Accordingly, prior to the scheduling of macrofungal collections in the Philippines, it is necessary to determine the climate type of the specific region of target.
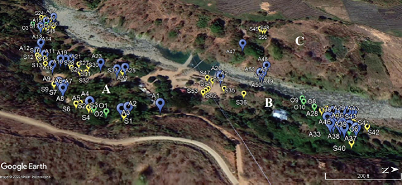 | Figure 2: Distribution of 116 collected macrofungi in the three collection sites (A, B, C), in the month of August (blue), September (yellow) and October (green). [Click here to view] |
Elevation or altitude is another important factor that contributes to the distribution of macrofungal species. Thus, the present study also assessed the macrofungal distribution in five elevation ranges. Macrofungi were found growing from the lowest elevation of 131 masl to the highest elevation of 177 masl. However, most of the macrofungi were documented at 141–150 masl (50.00%), followed by 151–160 masl (40.28%). This is because of the availability of substrates in the moderate vegetation of the multistorey agroforest and the secondary forest, which are situated at a lower elevation and in close proximity to Canding River. On the other hand, the highest elevation range recorded the lowest macrofungal composition (8.33%). This elevated area was noted in collection site C, which has few trees and active agricultural activities. Similar to the observation of Arenas et al. [16], the number of macrofungal species in two highest elevation ranges (812–869 masl and 870–927 masl) in Mt. Maculot significantly decreased, which is attributed to the stress vegetation and disturbed habitat due to hikers and tourists activities. It is hypothesized in the present study that the majority of macrofungi can be found in the highest elevation, since high elevation has a low temperature, which is favorable for macrofungal growth. But surprisingly, the highest elevation showed the lowest macrofungal composition. It is safe to mention; therefore, even at the highest elevation that has favorable climatic conditions, the proliferation of many macrofungi could still be impeded because of the no to low vegetation as a source of cellulosic-rich substrate and the disturbed natural habitat due to various human activities.
Altogether, the distribution of macrofungal species is not just affected by collection sites, collection time, and elevation itself but most importantly by the climatic conditions, availability of substrates, and man-made disturbances, which the three factors are comprised of. Moreover, the distribution is also accounted for the natural and unique responses of macrofungi to seasonal climatic changes.
3.3. Taxonomic Listing of Macrofungi
After the field assessment and documentation of the natural habitat and physical distribution of macrofungi, morphological identification of macrofungal specimens was carried out and a taxonomic checklist was prepared. The 116 collected macrofungi were taxonomically classified under two classes, 27 families, 46 genera, and 72 species (Table 2). Most of the macrofungi species belong to class Basidiomycetes and only three species were under class Ascomycetes, and these belong to Xylariaceae. Among Basidiomycetes, Agaricaceae had the most number of species, followed by Polyporaceae, Marasmiaceae, and Psathyrellaceae. However, Agaricus, Auricularia, and Ganoderma were the genera with the highest number of species. The species with the most number of collected macrofungal specimens were Trametes hirsuta (6), Auricularia polytricha (5), Favolus sp.1 (5), Hexagonia tenuis (5), and Schizophyllum commune (4). Notably, Favolus sp.1 and T. hirsuta occurred in all collection sites, while Xylaria polymorpha, Ganoderma lucidum, Earliella scabrosa, and S. commune appeared in all collection months. No common species were observed in all elevation ranges.
Out of 72 species, 33 species were morphologically identified down to the genus level and only and 39 species were identified down to the species level. To the best of our knowledge, among 39 species identified down to the species level, 13 were considered as newly recorded macrofungi in the Philippines. These include Gymnopilus lepidotus, Clitopilus prunulus, Geastrum schmidelii, Termitomyces microcarpus, Gerronema keralense, Hydropus marginellus, Pluteus multiformis, Volvariella dunensis, Favolus emerici, Coprinellus pakistanicus, Coprinopsis musae, Serpula similis, and Deconica coprophila. No taxonomic works have been done or reported yet regarding the above-mentioned species. At present, it is too early to mention whether macrofungal species identified down to the genus level only is a new record or not. Therefore, confirmation of the species identity using the molecular approach is very necessary, which is under current investigation in our laboratory. It is expected that, after the molecular identification of the 33 species, some of these can also be considered in the list of a new record of Philippine macrofungi.
 | Table 2. Taxonomic positions of the 72 species of macrofungi. [Click here to view] |
Since the occurrence of macrofungi is considerably dependent on the different factors in their natural habitat, there is a great possibility of extinction. Thus, prior to their extinction, the cell lines of wild macrofungal species must be rescued through tissue culture technique with the intention to preserve these wild myco-resources and to harness their full potential in various applications. In the present work, mycelia of 14 macrofungal species were successfully cultured on potato dextrose agar medium, namely, A. polytricha, C. prunulus, Cyathus sp., F. emerici, Favolus sp., Ganoderma australe, Ganoderma gibbosum, G. lucidum, Ganoderma neo-japonicum, Gymnopilus sp., H. tenuis, Lentinus sp., Oudemansiella canarii, and T. hirsuta. Aside from being edible, these macrofungi could also be valuable sources of bioactive compounds with several biological activities [28]. Ganoderma lucidum, for instance, has been reported to exhibit antidiabetic, anticholesterolemia, antihypertensive, antitumor and is used to treat chronic hepatitis, arthritis, insomnia, bronchitis, asthma, gastric ulcer, and cancer [29]. For some nonpalatable and nonedible macrofungi, they could be used in the accumulation and remediation of heavy metals and other chemical contaminants [30,31] or in the production of important enzymes and metabolites [32].
4. CONCLUSION
Collectively, secondary forest and agroecosystem along Canding River in Sitio Canding are natural habitats of many macrofungal species belonging to two classes, 27 families, 46 genera, and 72 species. Their distribution is considerably affected by collection site, collection month, and elevation, which comprise varied climatic conditions (temperature, humidity, and rainfall), vegetation as substrate, and the presence of man-made disturbances. Molecular identification of 33 macrofungal species morphologically identified down to the genus level only is a current study in our laboratory. However, 13 species are considered newly recorded macrofungi in the Philippines, while 14 species are successfully tissue-cultured in culture media. The cultivation potential of these macrofungi and their promising potential as sources of nutritious food, effective natural alternatives for various diseases, and many biotechnological applications are of high interest for further investigation in order to establish their roles in the Philippine mushroom industry.
5. ACKNOWLEDGEMENTS
The primary author acknowledges sincerely the Department of Science and Technology-Science Education Institute (DOST-SEI) in the Philippines for the scholarship grant. The authors convey their appreciation to their field guides, Mr. Angelo Caldito and Mrs. Pilipina L. Capinding, and collection assistants, Mr. Eduardo Damaso, Mr. Marcelino Abon, Mr. Wilson Mendoza, and Mr. Leomark Parocha. The CLSU Tuklas Lunas Center and the Philippine Council for Health Research and Development are gratefully acknowledged.
6. CONFLICT OF INTEREST
The authors declare no conflicts of interest.
7. REFERENCES
1. Dulay RMR, Arenas MC, Kalaw SP, Reyes RG, Cabrera EC. Proximate composition and functionality of the culinary-medicinal tiger sawgill mushroom, Lentinus tigrinus (Higher basidiomycetes), from the Philippines. Int J Med Mushrooms 2014;16(1):85–94. CrossRef
2. Dulay RMR, Ray K, Hou CT. Optimization of liquid culture conditions of Philippine wild edible mushrooms as potential source of bioactive lipids. Biocatal Agric Biotechnol 2015;4:409–15. CrossRef
3. Dulay RMR, Cabalar AC, De Roxas MJB, Concepcion JMP, Cruz NE, Esmeralda M, et al. Proximate composition and antioxidant activity of Panaeolus antillarium, a wild coprophilous mushroom. Curr Res Environ Appl Mycol 2015;5(1):52–9. CrossRef
4. Eguchi F, Kalaw SP, Dulay RMR, Miyasawa N, Yoshimoto H, Seyama T, et al. Nutrient composition and functional activity of different stages in the fruiting body development of Philippine paddy straw mushroom, Volvariella volvacea (Bull.:Fr.) Sing. Adv Environ Biol 2015;9(22):54–65.
5. De Leon AM, Luangsa-ard JJD, Karunarathna SC, Hyde KD, Reyes RG, dela Cruz TEE. Species listing, distribution, and molecular identification of macrofungi in six Aeta tribal communities in Central Luzon, Philippines. Mycosphere 2013;4(3):478–94. CrossRef
6. Tayamen MJ, Reyes RG, Floresca EJ, Abella EA. Domestication of wild edible mushrooms as non-timber forest products resources among the Aetas of Mt. Nagpale, Abucay, Bataan: Ganoderma sp. and Auricularia polytricha. J Trop Biol 2004;3:49–51.
7. De Leon AM, Kalaw SP, Dulay RMD, Undan JR, Alfonzo DO, Undan JQ, et al. Ethnomycological survey of the Kalanguya indigenous community in Caranglan, Nueva Ecija, Philippines. Curr Res Environ Appl Mycol 2016;6(1):61–6. CrossRef
8. Lazo CRM, Kalaw SP, De Leon AM. Ethnomycological survey of macrofungi utilized by Gaddang communities in Nueva Vizcaya, Philippines. Curr Res Environ Appl Mycol 2015;5(3):256–62. CrossRef
9. Tadiosa ER, Agbayani ES, Agustin NT. Preliminary study on the Macrofungi of Bazal-Baubo Watershed, Aurora Province, Central Luzon, Philippines. Asian J Biodivers 2011;2:149–71. CrossRef
10. Dulay RMR, Maglasang CC. Species listing of naturally occurring mushrooms in agroecosystem of Barangay Bambanaba, Cuyapo, Nueva Ecija, Philippines. Int J Biol Pharm Allied Sci 2017;6(8):1459–72.
11. Culala JM, Dulay RMR. Species listing of naturally occurring mushrooms in Central Luzon State University, Science City of Munoz, Nueva Ecija, Philippines. Int J Biol Pharm Allied Sci 2018;7(10):1890–9. CrossRef
12. Guzman CDM, Baltazar MM, Sanchez AJI, Linsangan MG, Dulay RMR. Molecular identification of four wild higher basidiomycetes collected in Mt. Mingan, Gabaldon, Nueva Ecija, Philippines. J Biodivers Environ Sci 2018;13(6):46–51.
13. Liwanag JMG, Santos EE, Flores FR, Clemente RF, Dulay RMR. Species listing of macrofungi in Angat Watershed Reservation, Bulacan Province, Luzon Island, Philippines. Int J Biol Pharm Allied Sci 2017;6(5):1060–8.
14. Tadiosa ER. Some noteworthy species of wood-rotting fungi found in the forested hills of La Union Province, Northern Luzon, Philippines. UST J Grad Res 1998;25(2):55–8.
15. De Castro MEG, Dulay RMR. Macrofungi in multi-storey agroforestry systems in Mt. Makiling Forest Reserve, Los Banos, Laguna, Philippines. J Chem Biol Phys Sci 2015;5(2):1646–55.
16. Arenas MC, Tadiosa ER, Reyes RG. Taxonomic inventory based on physical distribution of macrofungi in Mt. Maculot, Cuenca, Batangas, Philippines. Int J Biol Pharm Allied Sci 2018;7(5):672–87. CrossRef
17. Arenas MC, Tadiosa ER, Alejandro GJD, Reyes RG. Macroscopic fungal flora of Mts. Palaypalay – Mataas na gulod protected landscape, Southern Luzon, Philippines. Asian J Biodivers 2015;6(1):1–22. CrossRef
18. Tadiosa ER, Briones RU. Fungi of Taal Volcano protected landscape, Southern Luzon, Philippines. Asian J Biodivers 2013;4:46–64. CrossRef
19. Daep NA, Cajuday LA. Mushroom diversity at Mt. Malinao, Albay. PSSN Nature News 2003;2:57.
20. Biadnes GCQ, Tangonan NG. Assessment of the biodiversity of basidiomycetous fungi, insects and orchids in midmontane forest of Mt. Apo, Mindanao. PSSN Nature News 2003;2:59.
21. Quimio TH. Common mushroom of Mt. Makiling. Museum of Natural History. University of the Philippines Los Banos, Laguna, Philippines, 2001.
22. McKnight KH, McKnight VB. Peterson’s field guide on mushrooms. Houghton Mifflin Company, New York, NY, 429p, 1987.
23. Kauserud H, Heegaard RH, Boddy L, Hoiland K, Stenseth NC. Mushroom’s spore size and time of fruiting are strongly related: is moisture important? Biol Lett 2011;7:273–6. CrossRef
24. Chang ST, Miles PG. Edible mushrooms and their cultivation. CRC Press, Boca Raton, FL, 1989.
25. Lin Z. Grass (Juncao). Mushroom grower's handbook 1: oyster mushroom cultivation. Mush World-Heineart Inc., Seoul, Korea, pp 107–13, 2004.
26. Stamets P. Growing gourmet and medicinal mushrooms. Ten Speed Press, Berkeley CA, 1993.
27. Ramel DMB. Species listing and mycophagy of macrofungi found in Barobbob Watershed, Bayombong, Nueva Vizacya, Cagayan Valley Region. Master of Science thesis. Central Luzon State University, Science City of Munoz, Philippines, 2018.
28. Jikai L. Biologically active substances from mushrooms in Yunan, China. Heterocycles 2002;57:157–67. CrossRef
29. Borchers AT, Keen CL, Gershwin ME. Mushooms, tumors, and immunity: an update. Exp Biol Med 2004;229(5):393–406. CrossRef
30. Damodaran D, Vidya Shetty K, Raj Mohan B. Uptake of certain heavy metals from contaminated soil by mushroom-Galerina vittiformis. Ecotoxicol Environ Saf 2014;104:414–22. CrossRef
31. Dulay RMR, De Castro MEG, Coloma NB, Bernardo AP, Dela Cruz AG, Tiniola RC, et al. Effects and myco-accumulation of lead (Pb) in five Pleurotus mushrooms. Int J Biol Pharm Allied Sci 2015;4(3):1664–77.
32. Camassola M. Mushrooms – the incredible factory for enzymes and metabolites productions. Ferment Technol 2013;2(1). CrossRef