INTRODUCTION
Breast cancer ranks in second amongst all the prevalent cancer deaths reported globally [1]. Among Indian population, breast cancer is reported to be the highest with death ratio of 2:1 (out of two newly diagnosed breast cancers in women one dies) [2]. Despite the significant therapies available, such as hormone therapy, radiotherapy, chemotherapy, and surgery for the treatment of the breast cancer, their side effects and the chances of reoccurrence of the malignancy or metastatic cancer still projects a huge risk [3]. Therefore, there has been a hunt for the novel anticancer agent from the natural products which could endure as an alternative medicine for the treatment of cancer [4,5]. Some of the potent anticancer drugs, such as vinca alkaloids, vincristine, and vinblastine, have set up the foundation for search of several plant-based drugs [6]. The development of paclitaxel analogs was found to be potentially effective against breast cancer and Kaposi sarcoma, but in some duration of time, it was reported to be non productive in several cases [7]. Hence, there is an immediate need for the development of an effective drug which should be significantly effective in treatment of breast cancer but nontoxic to healthy cells of individuals. The identification of phytobioactive compounds from the medicinal plants against cancers with lower side effects has been the need of the hour in the recent decades [8]. Solanum macranthum (Dunal) is an ornamental plant belonging to Solanaceae family a native of Brazil and also found in different regions of Indian Territory displaying spectacular purple and white flowers has been undertaken for the study to unmask its potential against breast cancer [9]. The phyto-compounds isolated from Solanaceae family have been reported to possess several medicinal values including anticancer property [10]. Some species of Solanaceae have been traditionally used as folk medicines in treatment of various diseases. The current scientific research undertaken focused on aqueous extract of S. macranthum fruit to identify its cytotoxic properties against human breast cancer cell line MDA-MB-231.
2. MATERIALS AND METHOD
2.1. Chemical procured
Analytical grade chemicals were procured and used for the analysis. Acetic acid, Ammonium Hydroxide, Gallic acid monohydrate, and Folin–Ciocalteu reagent (FCR) were obtained from SDFCL Laboratories Pvt. Ltd (Mumbai, India). Camptothecin was obtained from Sigma-Aldrich. Dulbecco modified eagles medium (DMEM), MTT [3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide], Dimethylsulfoxide (DMSO), and Fetal Bovine Serum (FBS, Brazil, EU Approved) were obtained from Hi media Laboratories Pvt. Ltd.
2.2. Extraction of phytoconstituents
The S. macranthum fruit was collected from the medicinal garden of K.L.E’s P.C Jabin Science College Hubballi, Karnataka, India and shade dried. Dr. Harsha Hegde, ICMR—National Institute of Traditional Medicine taxonomically authenticated the plant with herbarium accession number RMRC-1402. The fruit appears oval in shape with a hard calyx on it. 200 g of dried fruit and 10 g of the fruit calyx were subjected for sequential extraction separately using four different solvents based on their increasing polarity for 144 hours, i.e., Petroleum Ether, Chloroform, methanol, and aqueous for 144 hours [10]. The extracts from each solvent were filtered and dried at room temperature. For the current study, aqueous extract has been used.
2.3. Analysis of the phytoconstituents
The dried aqueous extract of fruit and calyx was analyzed for the presence of various phytoconstituents, such as alkaloids, flavonoids, glycosides, phenols, saponins, tannins, terpenoids, and sterols separately using standard methods [11]. Since the aqueous fruit extract of S. macranthum was found to reveal more positive phytoconstituents in the preliminary qualitative analysis than the calyx of the fruit; therefore, further experimental study was executed using the aqueous fruit extract.
2.4. Total alkaloid content
The dried aqueous fruit extract of 1.5 g was added into 20% of acetic acid and the solution was incubated for 4 hours at 37°C. Furthermore, the solution was filtered and was reduced to its one fourth of its volume on water bath. Once the solution was cooled, concentrated ammonium hydroxide was added to it, in drop wise until the precipitation ceased. Using pre-weighed filter paper, the solution was filtered and dried. The percentage of crude dry weight of the alkaloid was determined by the formula [12]. The results were analyzed in triplicate.
2.5. Total phenol content
The total phenol present in aqueous fruit extract was estimated using the Folinsphenols reagent method described by Siddhuraju et al. [13] with slight modification. Aqueous extract of 0.5 ml (700 μg) was diluted to 1 ml using distilled water. 0.5 ml of Folins-phenols reagent and 2.5 ml of 20% sodium carbonate were added to the solution and incubated at 37°C in dark until colour develops. The absorbance was measured at 725 nm (Labman UV Visible Spectrophotometer: LMSP-UV1200PC) and total phenol content was determined and expressed as mg/g Gallic Acid Equivalent (GAE) using calibration curve where Gallic acid (50–200 μg/ml) is used as a standard and the results were analyzed in triplicate [13].
2.6. Cytotoxic effect of S. macranthum aqueous extract on MDA-MB-231 cell line
Breast cancer MDA-MB-231 cell lines were procured from National Centre for Cell Science (NCCS), Pune, India. The trypsinized monolayer cells were subcultured in DMEM in which 10% FBS was added, approximately 20,000 cells (200 μl) were seeded in 96-microtitre plate and the cells were incubated in 5% CO2 atmosphere at 37°C for 24 hours. 200 μl of different concentration of aqueous extract of the fruit and standard drug camptothecin was added to the partial monolayer (aspirated) of MDA-MB-231 cell suspension. Further the incubation was carried out in 5% CO2 atmosphere at 37°C for 24 hours. Media containing the drug was aspirated and MTT reagent (10%) was added to each well of the microtitre plate followed by 3 hours of incubation in 5% CO2 atmosphere at 37°C. after incubation 100 μl DMSO was added where solubilisation of formazan was observed. The percentage of inhibition was calculated and the results were analyzed in triplicate (n = 3), by measuring absorbance of microtitre plate at 570 and 630 nm using enzyme-linked immunosorbent assay reader (Biobase-EL10B) [14].
2.7. Statistical analysis
All the tests were carried out in triplicates (n = 3) and statistically analyzed and is presented as mean ± SE. Further Tukey t-test was carried out and statistical significance of the result was estimated were in Single * refers to p ≤ 0.05 and ** refers to p ≤ 0.01.
 | Table 1: The extracted yield of the S. macranthum aqueous fruit and calyx extract. [Click here to view] |
3. RESULTS AND DISSCUSSION
The total yield of aqueous crude extract of fruit and calyx under the controlled parameters was found to be 15.04 g for 200 g of dried fruit and 1.303 g for 10 g of calyx (Table 1). The qualitative analysis of the aqueous extract was found to be positive for different phytoconstituents which is depicted in Table 2. Secondary metabolites present in most of the plant species naturally, comprise vivid pharmacological properties in them, because of which these metabolites have gained a wide importance in the field of medicine [15]. As per the earlier reports, alkaloids and phenols are the secondary metabolites present in many in the species of the plant kingdom. Some of the pharmacological therapeutic properties of these metabolites in the Solanum species are reported to display potentially antiinflammatory, antitumor, anticholinergic antiviral, antihypertensive, antiulcer, analgesic, antioxidant, regulating carcinogen metabolism, oncogenesis expression, inhibiting DNA binding and cell adhesion [16,17]. The total alkaloid content present in aqueous extract of the S. macranthum was determined using the gravimetric analysis method described by Senguttuvan et al. [12] with slight modification. The total alkaloid content present in the aqueous extract of the fruit was calculated to be 7.58%. Using Gallic acid as a standard, the total phenol content present in the aqueous extract of the S. macranthum fruit was estimated to be 158.774 mg/GAE; this was calculated using the calibration curve (Fig. 1 and Table 3).
 | Table 2: The table depicts the phytoconstituents present in the S. macranthum aqueous fruit and calyx extract. [Click here to view] |
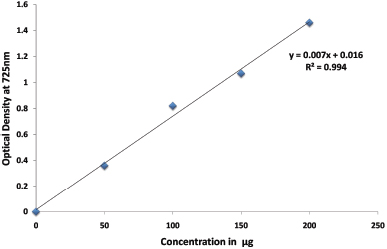 | Figure 1: Depicts the calibration curve of Gallic acid standard for the Determination of total phenol content of aqueous extract of S. macranthum fruits. [Click here to view] |
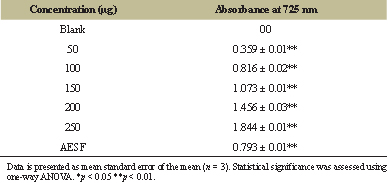 | Table 3: Total phenol content of aqueous extract of S. macranthum fruit (AESF). [Click here to view] |
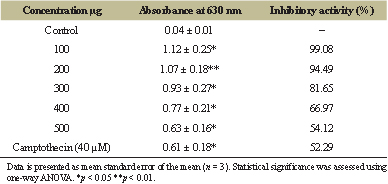 | Table 4: Cytotoxic effect of aqueous extract of S. macranthum fruit on MDA-MB-231 cell line. [Click here to view] |
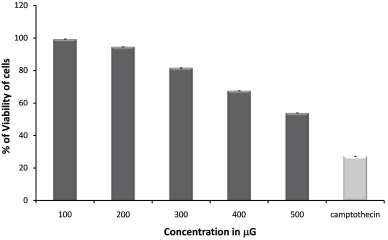 | Figure 2: Depicts cytotoxic effects of S. macranthum fruits aqueous extract on MDA-MB-231 cell line, concentrations (100–500 μg/ml) in triplicate (standard deviations in parentheses); Human breast cancer cell line IC50= 525.59 μg/ml; negative controls, medium and DMSO had no effects in the assay; Reference compound camptothecin at 40μM concentration. [Click here to view] |
Evaluation of anticancer property of the plant will enable to identify the intrinsic toxicity of the plant and the effects of acute overdose [18,19]. Solanum plant species have been well documented for their various therapeutic properties, including anti-inflammatory, antidiabetic, antioxidant, and also anti-cancer activity [16,17]. The bioactive compounds, such as glycoalkaloids, solamargine, solasodine, and solasonine, present in these species have been reported to possess anticancer activity and correlate to the toxic effect exhibited by them.
MTT assay is being frequently used as in vitro method to determine the cytotoxicity of the crude extract as well as isolated compounds. The MTT assay is mainly based on the reduction of tetrazolium salts by the metabolically active cells to form a purple colored formazan product by the action of dehydrogenase enzymes [20,21]. The earlier reports on the essential oils extracted from the S. macranthum fruit, had 79.39% cytotoxic effect at 250 μg/ml on Hs 578T cell line and 47.43% on PC-3 cell line at 100 μg/ml [22]. In the present study MDA-MB-231 breast cancer cell line was used to evaluate in vitro cytotoxic potential of aqueous extract of S. macranthum fruit. The extract was found to be very potent to inhibit the growth of MDA-MB-231 breast cancer cell line at low concentration, i.e., at 50 μg/ml concentration and toxic effect was elevated with increased concentration and the IC50 value was calculated to be 525.59 μg/ml (Table 4, Figs. 2 and 3). The cytotoxic effect of the aqueous extract was similar to that of standard camptothecin at 40μM.
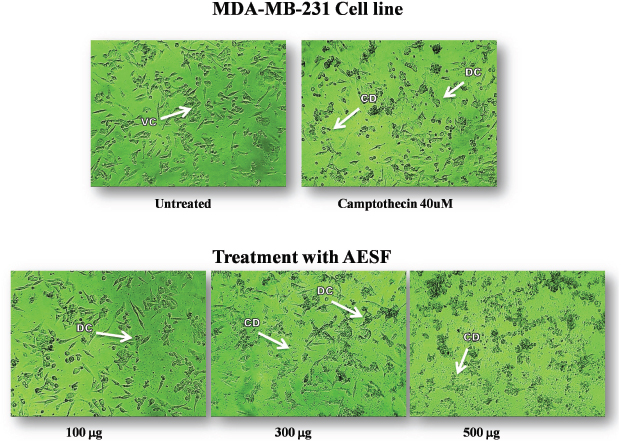 | Figure 3: The figure shows the concentration dependent toxic effect of Aqueous extract of S.macranthum fruits (AESF) on MDA-MB-231 Breast cancer cell line after 24hrs of incubation. VC:- Viable Cells, DC:- Dead Cells, CD:- Cell Debris. [Click here to view] |
4. CONCLUSION
In conclusion, the aqueous extract of S. macranthum fruit tends to possess potential anticancer property on MDA-MB-231 Breast cancer cell line. The phyto constituents, such as alkaloids, phenols, and saponins present in the extract may contribute to its cytotoxic property. Thus, further investigation need to be performed to identify the pharmacological bioactive compound present in the aqueous extract of S. macranthum fruit to assess its anticancer effects in in vivo models.
ACKNOWLEDGMENT
The authors would like to acknowledge Department of Biotechnology Karnatak University Dharwad and P.C Jabin Science College Hubballi for providing the laboratory facility to carry out the research. The authors would also like to acknowledge the support rendered by Cytxon Biosolutions Pvt Ltd Hubballi.
REFERENCES
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. CrossRef
2. Kulkarni BB, Kulkarni SS, Hallikeri UR, Patil BR, Gai PB. Decade of breast cancer-trends in patients profiles attending tertiary cancer care center in south India. Asian J Epidemiol 2012;5:103–3. CrossRef
3. Jayakumar K, Murugan K. Purified solasodine and caulophyllumine: a from Solanum mauritianum Scop. against MCF-7 breast cancer cell lines in terms of cell growth. Cell cycle and apoptosis. J Pharmacogn Phytochem 2017;6:472–8.
4. Pratumvinit B, Srisapoomi T, Worawattananon P, Opartkiattikul N, Jiratchariyakul W, Kummalue T. In vitro antineoplastic effect of Ficus hispida L. plant against breast cancer cell lines. J Med Plants Res 2009;3:255–61.
5. Elkady AI, Abuzinadah OA, Baeshen NA, Rahmy TR. Differential control of growth, apoptotic activity, and gene expression in human breast cancer cells by extracts derived from medicinal herbs Zingiber officinale. J Biomed Biotechnol 2012;2012:1–14. CrossRef
6. Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 2004;74:2157–84. CrossRef
7. Kamalanathan D, Natarajan D. Anticancer potential of leaf and leaf-derived callus extracts of Aerva javanica against MCF-7 breast cancer cell line. J Cancer Res Ther 2018;14:321–7.
8. Lal M, Parasar NR, Singh AK, Akhtar MS. Potentiality of anticancer plant-derived compounds of North-East India. In Anticancer plants: Properties and Application 2018 pp. 77-89. CrossRef
9. Yadav R, Rathi M, Pednekar A, Rewachandani Y. A detailed review on Solanaceae family. Euro J Pharm Med Res 2016;3:369–78.
10. Kalebar VU, Hoskeri JH, Hiremath SV, AB Kalebar RV, Sonappanavar KL, Agadi BS, et.al., Pharmacognostical and phytochemical analysis of Solanum macranthum (Dunal) Fruits. J Pharmacogn Phytochem 2019;8:284–90.
11. Kumar M, Mondal P, Borah S, Mahato K. Physico-chemical evaluation, preliminary phytochemical investigation, fluorescence and TLC analysis of leaves of the plant Lasia spinosa (Lour) Thwaites. Int J Pharm Pharm Sci 2013;5:306–10.
12. Senguttuvan J, Paulsamy S, Karthika K. Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. for in vitro antioxidant activities. Asian Pac J Trop Biomed 2014;4:59–67. CrossRef
13. Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem 2003;51:2144–55. CrossRef
14. Alley MC, Scudiero DA, Monks A, Czerwiski MJ, Shoemaker RH, Boyd MR. Variation of an automated microculture tetrazolium assay (MTA) to assess cell growth and drug sensitivity of human tumor cell lines. Proc Am Assoc Cancer Res 1986;389.
15. Murugesan S, Lakshmanan DK, Arumugam V, Alexander RA. Nutritional and therapeutic benefits of medicinal plant Pithecellobium dulce (Fabaceae): a review. J Appl Pharm Sci 2019;9:130–9. CrossRef
16. Alves de Almeida AC, de-Faria FM, Dunder RJ, Manzo LP, Souza-Brito AR, Luiz-Ferreira A. Recent trends in pharmacological activity of alkaloids in animal colitis: potential use for inflammatory bowel disease. Evid Based Complement Altern Med 2017;2017:1–24. CrossRef
17. Fekry MI, Ezzat SM, Salama MM, Alshehri OY, Al-Abd AM. Bioactive glycoalkaloides isolated from Solanum melongena fruit peels with potential anticancer properties against hepatocellular carcinoma cells. Sci Reports 2019;9:1–11. CrossRef
18. Matias Ld, Rocha JA, Royo Vd, Menezes EV, Júnior AF, de Oliveira DA. Phytochemistry in medicinal species of Solanum L. (Solanaceae). Phcog Res 2019;11:47–50. CrossRef
19. Rahman MA, Akhtar J, Siddiqui S, Arshad M. Evaluation of cytotoxic potential and apoptotic effect of a methanolicextract of Bauhinia racemosa Lam against a human cancercell line, HeLa. Eur J Integr Med 2016;8:513–8. CrossRef
20. Morshed MA, Uddin A, Rahman A, Hasan T, Roy S, Amin AA,et al. In vitro antimicrobial and cytotoxicity screening of Terminalia arjuna ethanol extract. Int J Biosci 2011;1:31–8.
21. Rai Y, Pathak R, Kumari N, Sah DK, Pandey S, Kalra N, et al. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci Rep 2018;8:1–15. CrossRef
22. Essien EE, Ogunwande IA, Setzer WN, Ekundayo O. Chemical composition, antimicrobial, and cytotoxicity studies on S. erianthum and S. macranthum essential oils. Pharm Biol 2012;50:474–80. CrossRef