1. INTRODUCTION
Cordyceps militaris, a medicinal and edible fungus with a long history in traditional Asian medicine, has attracted considerable scientific interest due to its diverse bioactive compounds, particularly polysaccharides (PSs). These PSs exhibit a range of functional properties, including immunomodulatory, antioxidant, and anticancer activities [1]. Notably, their antioxidant and prebiotic properties have emerged as key research areas, offering potential health benefits in preventing oxidative stress-related diseases and promoting gut microbiota balance. However, the functional mechanisms and bioactivity of C. militaris polysaccharide (CMP) extracts remain incompletely understood, necessitating further investigation.
Fungal PSs, high-molecular-weight carbohydrates composed of monosaccharide units linked by glycosidic bonds, are crucial to C. militaris pharmacological potential. Major PSs found in this fungus include β-glucans, α-glucans, galactomannans, and heteropolysaccharides containing rhamnose, arabinose, xylose, mannose, glucose, and galactose [2]. The extraction method, such as hot water extraction (HWE), acid/alkali treatment, or enzymatic hydrolysis, significantly influences the yield, structure, and bioactivity of these PSs [1]. β-glucans, consisting of β-(1→3)- and β-(1→6)-linked glucose units, exhibit strong immunomodulatory and antioxidant properties [3]. Branched PSs with heteropolysaccharide backbones also enhance solubility and biological functions. Advanced extraction techniques, including ultrasound-assisted, enzymatic, and microwave-assisted extraction, and purification methods, such as ion-exchange and gel filtration chromatography, improve PS recovery and isolate fractions with enhanced bioactivity [4].
Oxidative stress is a critical factor in various diseases, including neurodegenerative disorders, cardiovascular diseases, and cancer. CMPs exert antioxidant effects through multiple mechanisms, including direct free radical scavenging (ABTS, 2,2-diphenyl-1-picrylhydrazyl, hydroxyl radicals, and superoxide anions) [5], enhancement of endogenous antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase) [6], and reduction of lipid peroxidation [7]. However, variations in extraction methods and structural differences can significantly influence their efficacy [8]. Therefore, understanding the structure-function relationship of CMPs in mitigating oxidative stress is crucial for their therapeutic applications.
Beyond their antioxidant potential, CMPs are increasingly recognized as prebiotics, selectively stimulating the growth and activity of beneficial gut microbiota. The gut microbiome plays a pivotal role in human health [9]. While prebiotics like inulin and fructooligosaccharides (FOSs) are well-studied, the prebiotic effects of fungal PSs remain relatively underexplored. Recent studies suggest that CMPs can enhance probiotic proliferation, modulate gut microbiota composition, and improve gut barrier integrity [10-12]. However, the precise mechanisms, including fermentation characteristics and short-chain fatty acid (SCFA) production, require further elucidation.
This research aims to comprehensively assess the antioxidant and prebiotic activities of PS extracts from C. militaris mycelium. By employing PS sequential extraction and bioactivity assays, this study will establish a correlation between CMP morphological features and functional properties. Furthermore, the prebiotic potential of CMPs will be evaluated through in vitro gut microbiota fermentation, providing insights into their role in modulating microbial ecology. The findings will contribute to the growing body of evidence supporting the application of fungal PSs as natural antioxidants and gut microbiota modulators, offering novel strategies for functional food and nutraceutical development.
2. MATERIALS AND METHODS
2.1. Microorganisms and Reagents
C. militaris strain was cultivated at the Fungal Biotechnology Laboratory, Faculty of Biology and Environmental Science, University of Science and Education – The University of Danang.
Probiotic strains Lactiplantibacillus plantarum WCFS1, Lactiplantibacillus pentosus NH1, Pediococcus acidilactici NDB8, Lacticaseibacillus casei-01, and Bifidobacterium animalis YC381, along with the pathogenic strain Escherichia coli American Type Culture Collection (ATCC) 85922, were used to assess the prebiotic activity of the CMP extracts.
Commercial FOS was obtained from Novaco Pharmaceutical Joint Stock Company (Hanoi, Vietnam).
2.2. PS Extraction and Mycelial Preparation
2.2.1. Mycelial cultivation
Potato Dextrose Broth (100 mL) was dispensed into 500 mL Erlenmeyer flasks and sterilized by autoclaving at 121°C for 30 min (Hirayama HV 110 L, Japan). Following cooling, each flask was inoculated with a uniform area of C. militaris mycelium from a first-generation seed culture. The flasks were incubated statically in the dark at 22°C for 20 days. Mycelia were then harvested and dried to a constant weight at 50°C.
2.2.2. PS sequential extraction
PSs were extracted from the dried C. militaris mycelia using a sequential solvent extraction method, based on the protocols of Peng et al. (2023) and Yu et al. (2024), with minor modifications [13,14]. The detailed extraction procedure is depicted in Figure 1.
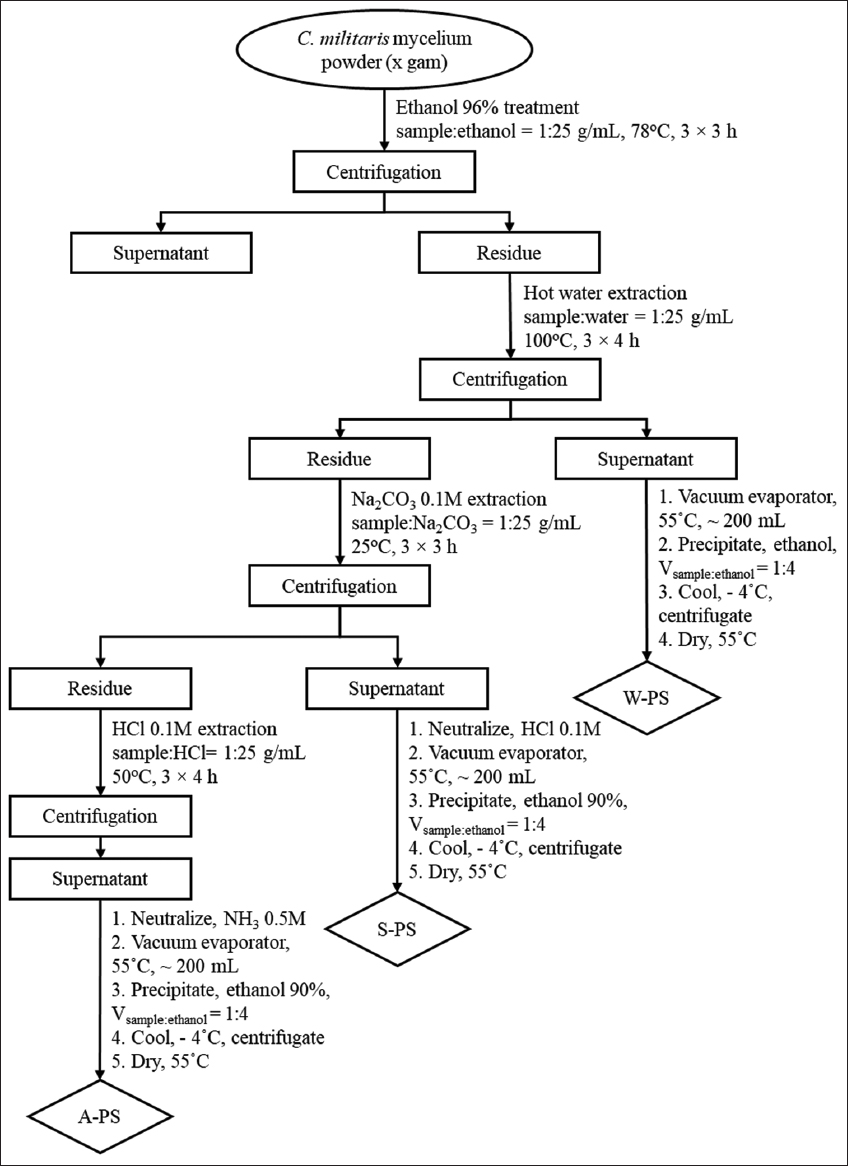 | Figure 1: Flow chart of polysaccharide sequential extraction procedure from Cordyceps militaris mycelium. W-PS: Water extraction; S-PS: Na2CO3 extraction, and A-PS: HCl extraction. [Click here to view] |
2.3. Characterization of PS Extracts
The surface morphology of the PS extracts was visualized using a Jeol JSM IT-200 scanning electron microscope equipped with an energy dispersive X-ray element energy-dispersive X-ray spectrometer. Dried PS extracts were mounted onto sample stubs and coated with platinum, estimated coating thickness is about 3–5 nm, sputtering current of 30 mA, and sputtering time of 40s. Images were acquired at accelerating voltages of 15 kV and magnifications of ×1000, ×3000, ×5000, and ×10000 [15].
2.4. ABTS Radical Scavenging Assay
The ABTS radical scavenging activity was determined using the method described by Nenadis et al. (2004). The ABTS+ solution was prepared by mixing 7 mM ABTS with 2.45 mM K2S2O8 (1:1 v/v) and incubating in the dark for 16 h. The resulting solution was diluted 50-fold with ethanol to achieve an absorbance of 0.7 ± 0.05 at 734 nm. For the assay, 10 μL of PS extract was added to 990 μL of ABTS+ solution. After a 6-min incubation, the absorbance was measured at 734 nm [16]. Vitamin C was used as a positive control. The ABTS+ radical scavenging activity (%) was calculated using the following formula [17]:
I (%) = (A0−A1)/A0 × 100%
Where, A0 and A1 are the optical densities of blank and working samples.
2.5. Prebiotic Activity Evaluation
The growth-stimulating effect of PS extracts on probiotic strains was evaluated using modified De Man–Rogosa–Sharpe (MRS). The following treatments were employed: PGF (glucose-free MRS), PPS (PGF supplemented with 1 g/L PS extracts: W-PS, S-PS, and A-PS), PFOS (PGF supplemented with 1 g/L FOS), and PGlc (PGF supplemented with 1 g/L glucose). A ratio of 1% (v/v, ~106 CFU/mL) probiotic strains L. plantarum WCFS1, L. pentosus NH1, P. acidilactici NDB8, L. casei-01, and B. animalis YC381 was inoculated into these media and incubated at 37°C for 24 h. Cell density was determined by measuring the optical density at 600 nm (OD600) at 0 and 24 h [18].
The effect of PS extracts on the growth of E. coli ATCC 85922 was assessed using modified M9 media, which is specified for E. coli cultivation. The treatments were EGF (glucose-free M9), EPS (EGF supplemented with 1 g/L PS extracts), EFOS (EGF supplemented with 1 g/L FOS), and EGlc (EGF supplemented with 1 g/L glucose). E. coli ATCC 85922 was cultured at 37°C for 24 h, and cell density was determined by measuring OD600 at 0 and 24 h [19]. The prebiotic index (PI) was calculated using the following formula:
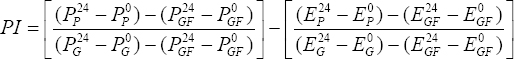 |
Where:
pP0and Pp24 and are the OD600 values of probiotic strains measured in media supplemented with 1 g/L prebiotic (PPS or PFOS) at 0 and 24 h, respectively.
pGF0 and P24GF are the OD600 values of probiotic strains measured in glucose-free media (PGF) at 0 and 24 h, respectively.
pG0 and pG24 are the OD600 values of probiotic strains measured in media supplemented with 1 g/L glucose (PG) at 0 and 24 h, respectively.
EP0 and EP24 are the OD600 values of E. coli measured in media supplemented with 1 g/L prebiotic (EPS or EFOS) at 0 and 24 h, respectively.
EG0 and EG24 are the OD600 values of E. coli measured in media supplemented with 1 g/L glucose (EGlc) at 0 and 24 h, respectively.
EGF0 and EGF24 are the OD600 values of E. coli measured in glucose-free media (EGF) at 0 and 24 h, respectively.
2.6. Quantification of SCFAs
SCFAs (acetic, propionic, and butyric acids) were quantified in the culture medium of probiotic strains after 24 h of incubation. The SCFAs were analyzed by high-performance liquid chromatography (HPLC) using an Agilent 1200 series HPLC system with a UV detector (Agilent, Germany) and a Phenomenex C18 column (Phenomenex, USA). Before HPLC analysis, the culture medium was filtered through a 0.20 μm cellulose acetate membrane. The mobile phase was acetonitrile and 5 mM sulfuric acid at a flow rate of 1 mL/min. We have described this in the revised manuscript [20].
2.7. Statistical Analysis
All data are presented as the mean ± standard deviation of three independent experiments. Statistical significance was determined using one-way analysis of variance followed by Tukey’s Honestly Significant Difference (HSD) test. Statistical analyses were performed using R for Windows version 4.3. A P < 0.05 was considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. PS Yield from C. militaris Mycelia
The sequential extraction of PS from C. militaris mycelia, using hot water (W-PS), sodium carbonate (S-PS), and hydrochloric acid (A-PS), yielded significantly different PS contents (Figure 2, Tukey’s HSD, P < 0.05). The HWE (W-PS) yielded the highest PS content at 81.35 ± 4.22 mg/g, followed by the sodium carbonate extract (S-PS) at 22.53 ± 1.84 mg/g, and the hydrochloric acid extract (A-PS) at 13.33 ± 3.46 mg/g [Figure 2].
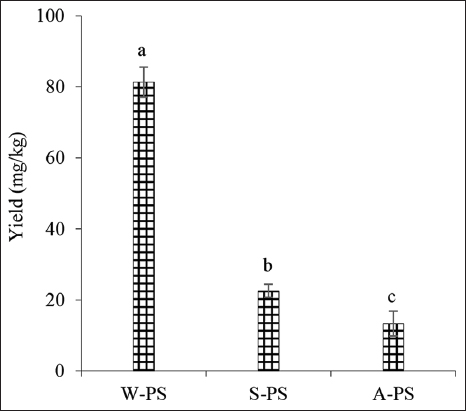 | Figure 2: Yield of polysaccharides (PS) extracted from Cordyceps militaris mycelium. W-PS (water), A-PS (HCl), and S-PS (Na2CO3) extracts. Significant differences are indicated by different letters (Tukey’s Honestly Significant Difference, P < 0.05). [Click here to view] |
These data highlight that while hot water effectively extracts a significant portion of PS, substantial amounts remain insoluble. The subsequent alkaline (S-PS) and acidic (A-PS) extractions demonstrate the ability of these solvents to disrupt glycosidic bonds and release residual PS, as previously reported [21].
The observed PS yields are consistent with the known variability of PS content in Cordyceps species. Previous studies have documented PS content ranging from 3% to 8% in C. sinensis fruiting bodies [22] and 157.3 mg/g in mycelia [23]. In C. militaris, reported values include 260.64 mg/g in fruiting bodies and 389.47 mg/g in mycelia, with variations dependent on extraction methods [4,24].
These variations underscore the significant impact of cultivation parameters and extraction techniques on PS yield. Factors such as substrate selection [25], light exposure, and biotic elicitors [26] influence PS biosynthesis. Furthermore, extraction method optimization, including solvent choice, temperature, and time, is crucial for maximizing PS recovery and maintaining its structural integrity [4,27].
The high W-PS yield in this study suggests that HWE is an efficient primary method. HWE is widely regarded as an effective method for extracting PSs from medicinal mushrooms due to its simplicity, safety, and ability to preserve the structural integrity and bioactivity of the PSs. Many bioactive PSs, such as β-glucans, are water-soluble and can be efficiently extracted using hot water without the need for harsh chemicals. Studies have shown that HWE can yield significant amounts of PSs with potent antioxidant and immunomodulatory activities. For instance, a study on Lentinula edodes demonstrated that HWE yielded 8.44% PSs, which exhibited notable immunostimulatory effects [28]. Moreover, HWE is considered more environmentally friendly and cost-effective compared to acid or alkaline extraction methods, which may degrade PS structures and require additional purification steps [29]. However, the additional recovery of PS through alkaline and acidic extractions indicates the presence of diverse PS fractions with potentially distinct bioactivities. Further characterization of these fractions is essential for the comprehensive utilization of CMP.
3.2. Scanning Electron Microscopy (SEM) Analysis of PS Extracts
SEM analysis revealed distinct morphological features among the PS extracts obtained by different methods. All three extracts exhibited dense shapes and small, aggregated clumps, suggesting strong intermolecular interactions, possibly due to branching or electrostatic forces [30].
The water-extracted PS (W-PS) displayed relatively large, well-defined particles with rough surfaces, indicating minimal structural degradation and the formation of larger aggregates. In contrast, the Na2CO3-extracted PS (S-PS) exhibited more fragmented particles with irregular shapes, likely due to partial structural breakdown induced by the alkaline environment. The HCl-extracted PS (A-PS) showed the finest particles with significant agglomeration, suggesting substantial hydrolysis and chain fragmentation in the acidic environment [Figure 3].
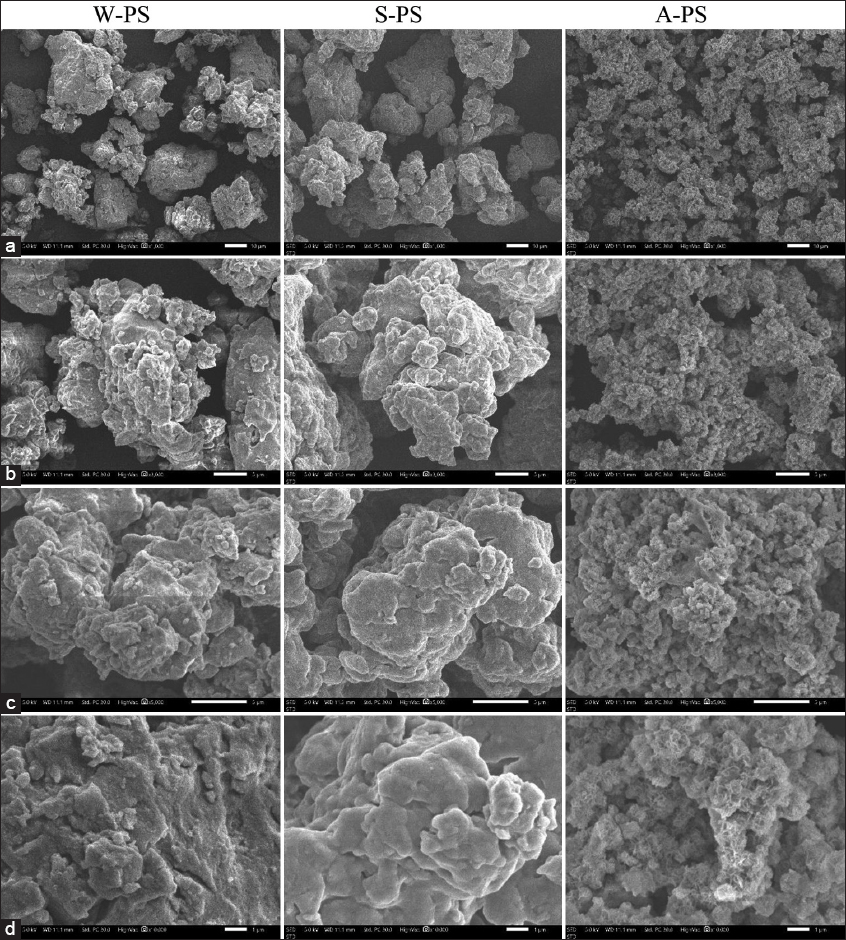 | Figure 3: Scanning electron micrographs of Cordyceps militaris polysaccharide (PS) extracts at magnifications of ×1000 (a), ×3000 (b), ×5000 (c), and ×10,000 (d). W-PS: Water extract, S-PS: Na2CO3 extract, A-PS: HCl extract. [Click here to view] |
These observations demonstrate that the extraction method significantly influences the morphology of CMP, which likely affects its physicochemical properties. A study on Polygonatum sibiricum polysaccharides (PSPs) further supports this notion, showing that different extraction methods (hot water, alkali, ultrasound, enzyme, microwave, and freeze-thaw) yielded PSPs with varying molecular weights, rheological properties, and biological activities [31]. For example, alkali-extracted PSPs exhibited higher molecular weights and improved rheological properties, while enzyme- and freeze-thaw-extracted PSPs showed enhanced lipid-lowering activity due to lower molecular weights. Ultrasound-assisted extraction of PSs from Sinopodophyllum hexandrum reduced molecular weight, altered surface structure, and enhanced antioxidant activity without disrupting the primary structure [32]. Similarly, microwave-assisted extraction from Althaea officinalis roots produced PSs with uniform molecular weight and distinct surface morphology, contributing to improved antioxidant and antimicrobial activities [33].
The varying morphologies observed in our study suggest that the extraction method can be tailored to obtain PS fractions with specific structural characteristics and potentially desired bioactivities. Further characterization of the molecular weights and structural features of these extracts is necessary to fully elucidate the relationship between the extraction method and PS properties.
3.3. Antioxidant Capacity of PS Extracts: ABTS Radical Scavenging Activity
The ABTS radical scavenging activity of the PS extracts increased with concentration, ranging from 100 to 5000 μg/mL. At 5000 μg/mL, W-PS exhibited the highest scavenging activity (86.15%), followed by S-PS (81.51%) and A-PS (69.64%). The IC50 values were 2446.51 ± 24.34 μg/mL (W-PS), 2474.24 ± 2.78 μg/mL (S-PS), and 3273.33 ± 18.54 μg/mL (A-PS) [Table 1]. Ascorbic acid, used as a positive control, had an IC50 of 83.89 ± 0.39 μg/mL [Table 1]. The IC50 values of the CMP extracts are comparable to those reported for C. sinensis mycelial PS (2643.89 μg/mL) [34]. This is a common characteristic of natural-origin PSs, which typically lack strong redox-active functional groups such as phenolic or flavonoid moieties, the key constituents of standard antioxidants. Ascorbic acid acts as a direct antioxidant, rapidly neutralizing free radicals. In contrast, the strength of PSs lies in their versatility, high safety profile, and capacity for complex interactions with biological systems.
Table 1: ABTS radical scavenging activity (%) of PS extracts.
| PS extract concentration (µg/mL) | W-PS | S-PS | A-PS |
|---|---|---|---|
| 100 | 11.84±0.34 | 13.88±0.13 | 7.49±0.74 |
| 200 | 18.58±0.81 | 16.99±0.13 | 8.83±0.44 |
| 400 | 21.58±0.54 | 22.88±0.47 | 13.73±0.44 |
| 600 | 22.25±0.47 | 26.94±0.59 | 17.82±0.65 |
| 800 | 26.14±0.29 | 30.36±0.42 | 21.19±0.67 |
| 1000 | 31.37±0.91 | 32.59±0.37 | 24.54±0.34 |
| 2000 | 44.88±0.31 | 46.91±0.27 | 36.7±0.18 |
| 5000 | 86.15±0.79 | 81.51±0.56 | 69.64±0.51 |
The free radical scavenging activity of PSs is attributed to their ability to donate electrons or hydrogen atoms. Previous studies have shown that Cordyceps species, including C. sinensis, C. militaris, and C. cicadae, exhibit significant ABTS radical scavenging activity [35-37]. The composition of PSs, including monosaccharide types and glycosidic linkages, plays a crucial role. For example, Chen (2014) reported that C. sinensis PS, rich in Glc, Man, Gal, and Ara with α-glycosidic linkages, effectively scavenged hydroxyl and superoxide radicals [38]. The present study further suggests that Glc content, β-configurations, and ionic components contribute to the antioxidant activity of CMP extracts.
The antioxidant activity of PSs is influenced by various factors, including molecular weight, monosaccharide composition, functional groups, extraction methods, and structural characteristics. Lower-molecular-weight PSs often exhibit higher antioxidant activity [39]. Acidic PSs with higher uronic acid content demonstrate stronger ABTS radical scavenging activity compared to neutral PSs [40]. Functional groups, such as uronic acids and sulfates, enhance antioxidant activity by increasing electron density [41]. Extraction methods, such as ultrasonic-assisted extraction, can also influence antioxidant capacity [42]. Furthermore, structural features, including branching patterns and glycosidic linkages, play a significant role [43].
The observed differences in ABTS radical scavenging activity among the W-PS, S-PS, and A-PS extracts likely reflect variations in their structural characteristics and composition due to the different extraction conditions. Further investigation into the molecular weight, monosaccharide composition, and functional groups of these extracts is needed to fully understand their antioxidant mechanisms.
3.4. Prebiotic Activity of PS Extracts
Prebiotics are non-digestible food components that selectively promote the growth of beneficial microorganisms [44]. The prebiotic activity of PS extracts is attributed to their ability to be selectively metabolized by probiotics, thus stimulating their growth [45].
3.4.1. Effect of PS extracts on probiotic growth
As shown in Figure 4, probiotic strains exhibited the highest growth rates in media supplemented with glucose (PGlc) and FOS (PFOS), except for L. plantarum WCFS1, which showed higher growth rates in W-PS and A-PS supplemented media compared to PFOS. Notably, all probiotic strains grew better in W-PS and A-PS supplemented media than in S-PS supplemented or glucose-free (PGF) media. This suggests that PS extracts from C. militaris mycelium can serve as a carbon source for probiotics. The lower growth rates observed in S-PS-supplemented media may be due to the lower water solubility of this extract, hindering its utilization by probiotics.
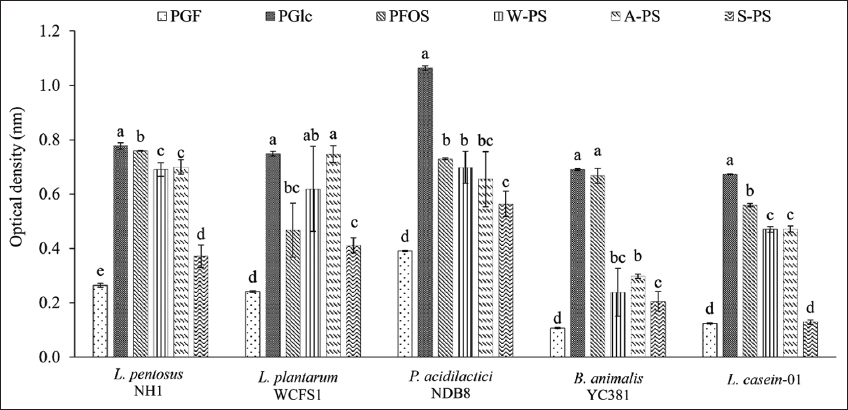 | Figure 4: Optical density (OD600) of probiotic strains grown in various media. PGF: Glucose-free MRS, PGlc: MRS supplemented with 1 g/L glucose, PFOS: MRS supplemented with 1 g/L fructooligosaccharide, W-PS: MRS supplemented with 1 g/L water extract, A-PS: MRS supplemented with 1 g/L HCl extract, S-PS: MRS supplemented with 1 g/L Na2CO3 extract. Different letters within the same strain indicate significant differences (Tukey’s Honestly Significant Difference, P < 0.05). [Click here to view] |
These findings are consistent with previous reports demonstrating the prebiotic potential of PSs. Phirom-On and Apiraksakorn (2021) showed that L. plantarum and L. casei strains can utilize cellodextrin, a glucose polymer derived from banana peel, as a carbon source [18]. Similarly, Song et al. (2022) reported that pectic PSs from soybean hulls, composed of glucose and galactose, exhibit prebiotic activity [46].
The variability in probiotics growth across different PS extracts may be attributed to differences in PS structure, the spectrum of hydrolytic enzymes produced by each probiotic strain, and the monosaccharide composition of the extracts. Lower-molecular-weight PSs are generally more readily utilized by probiotics [47], for instance, research on Litchi chinensis pulp PSs demonstrated that a fraction with a molecular weight of 16.96 kDa was more effectively utilized by probiotics compared to a higher-molecular-weight fraction, likely due to its lower molecular weight and higher arabinose and galactose content, which are favorable for probiotic growth [48]; and water solubility is also a critical factor, more water-soluble PSs have been associated with enhanced probiotic proliferation. PS exhibited higher solubility and lower viscosity might improve its dispersibility and accessibility to probiotic enzymes [49]. Water solubility indeed represents a decisive factor in determining the prebiotic efficacy of PS, as it directly influences their dispersibility in aqueous culture systems and the extent of interaction with probiotic cells. PS with higher solubility and lower viscosity can be more evenly dispersed in the medium, which not only facilitates enzymatic hydrolysis by probiotics but also increases the availability of fermentable monosaccharide units. Solubility enhances fermentability and selectively stimulates beneficial gut microbiota, while high viscosity can hinder microbial access to glycosidic linkages and reduce metabolic conversion rates. Therefore, the superior prebiotic performance observed with water-extracted C. militaris PS in the current study may partly be attributed to their improved solubility and lower viscosity, which promote efficient utilization by probiotic strains Furthermore, structural differences, such as glycosidic linkages, can affect prebiotic activity. For example, α-(1,3)-glucan from Pleurotus species showed varying growth-promoting effects on Bifidobacteria and Lactobacillus [50]. In the context of this study, although we did not yet characterize the specific glycosidic linkages of C. militaris polysaccharides, it is reasonable to propose that differences in extraction methods (water, acid, alkaline) may have altered linkage composition and branching structures, thereby influencing how effectively probiotics metabolized each extract. This could help explain why water-extracted PSs exhibited superior growth-promoting effects, as their structural configuration may have been more compatible with the enzymatic repertoires of the tested probiotic strains. Therefore, deeper studies on the properties of PS extracted from C. militaris mycelium such as molecular weight, monosaccharide composition, solubility, viscosity, and molecular bonding type need to be conducted to demonstrate and explain the above observations.
The results of this study suggest that W-PS and A-PS extracts from C. militaris are promising prebiotic candidates. Further characterization of the molecular weight, monosaccharide composition, and structural features of these extracts is warranted to optimize their prebiotic potential.
3.4.2. PI of PS extracts
The PI quantifies the selective stimulation of beneficial gut microbiota over pathogenic bacteria by a given carbohydrate. A higher PI indicates a stronger prebiotic effect [51].
As depicted in Figure 5, all PS extracts exhibited positive PI values across the tested probiotic strains. Notably, water-extracted PS (W-PS) generally yielded higher PI values compared to HCl-extracted (A-PS) and Na2CO3-extracted (S-PS) PS. L. pentosus NH1 showed the highest PI with W-PS (0.857) and A-PS (0.732), while L. plantarum WCFS1 exhibited the highest PI with A-PS (0.854) and W-PS (0.75), although this difference was not statistically significant. S-PS consistently resulted in low PI values, ranging from 0.051 in L. casei-01 to 0.342 in L. plantarum WCFS1. Remarkably, W-PS exhibited higher PI values than FOS in all tested probiotic strains.
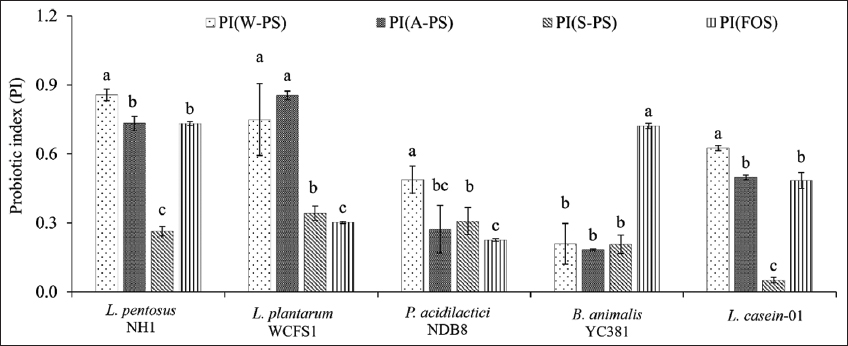 | Figure 5: Prebiotic index scores of probiotic strains grown in media supplemented with polysaccharide extracts. Different letters within the same strain indicate significant differences (Tukey’s Honestly Significant Difference, P < 0.05). [Click here to view] |
Furthermore, the PI varied among different probiotic strains for the same prebiotic. For instance, FOS yielded PI values of 0.732 and 0.722 in L. pentosus NH1 and B. animalis YC381, respectively, but only 0.302 and 0.225 in L. plantarum WCFS1 and P. acidilactici NDB8. This variability likely arises from differences in the hydrolytic enzyme systems and gene transport mechanisms among probiotic strains [52]. Genetic variations and permutations in these systems can lead to diverse abilities to utilize prebiotics.
The PI of PSs is influenced by several factors, including molecular structure, degree of polymerization, water solubility, fermentation rate, SCFA production, microbial selectivity, and processing methods. Shorter-chain oligosaccharides (FOS, GOS) generally exhibit higher PI values due to their ease of fermentation, while long-chain PSs (cellulose, resistant starch) show lower PI values [53]. Soluble fibers (inulin, pectins, and β-glucans) tend to have higher PI values compared to insoluble fibers (cellulose) due to their enhanced fermentability and SCFA production [54]. Effective prebiotics selectively stimulate beneficial bacteria (e.g., Lactobacillus and Bifidobacterium) while limiting pathogen growth [55]. Processing methods, such as enzymatic hydrolysis and heat treatments, can also influence PI [53].
The observed differences in PI values among the PS extracts and probiotic strains highlight the complex interplay between PS structure, microbial metabolism, and prebiotic activity. The high PI values of W-PS suggest its potential as a promising prebiotic candidate. Further characterization of the molecular structure, monosaccharide composition, and fermentation kinetics of these extracts is needed to fully elucidate their prebiotic mechanisms.
3.5. SCFA Production by Probiotics
SCFAs, primarily acetate, propionate, and butyrate, are produced by microbial fermentation of carbohydrates, including PSs, in the gastrointestinal tract. These organic acids play a crucial role in gut health, immune modulation, and metabolic processes [56].
As shown in Figure 6, acetic acid was the most abundant SCFA produced, ranging from 5044.35 to 7237.35 mg/kg, followed by butyric acid (3147.95 to 6634.99 mg/kg) and propionic acid (138.31–328.97 mg/kg). The production of acetic acid did not significantly differ among the PS extracts (W-PS, A-PS, S-PS) and FOS. However, propionic and butyric acid production was significantly higher in media supplemented with W-PS compared to A-PS, S-PS, and FOS, except for B. animalis YC381, which exhibited higher propionic and butyric acid production in FOS-supplemented media.
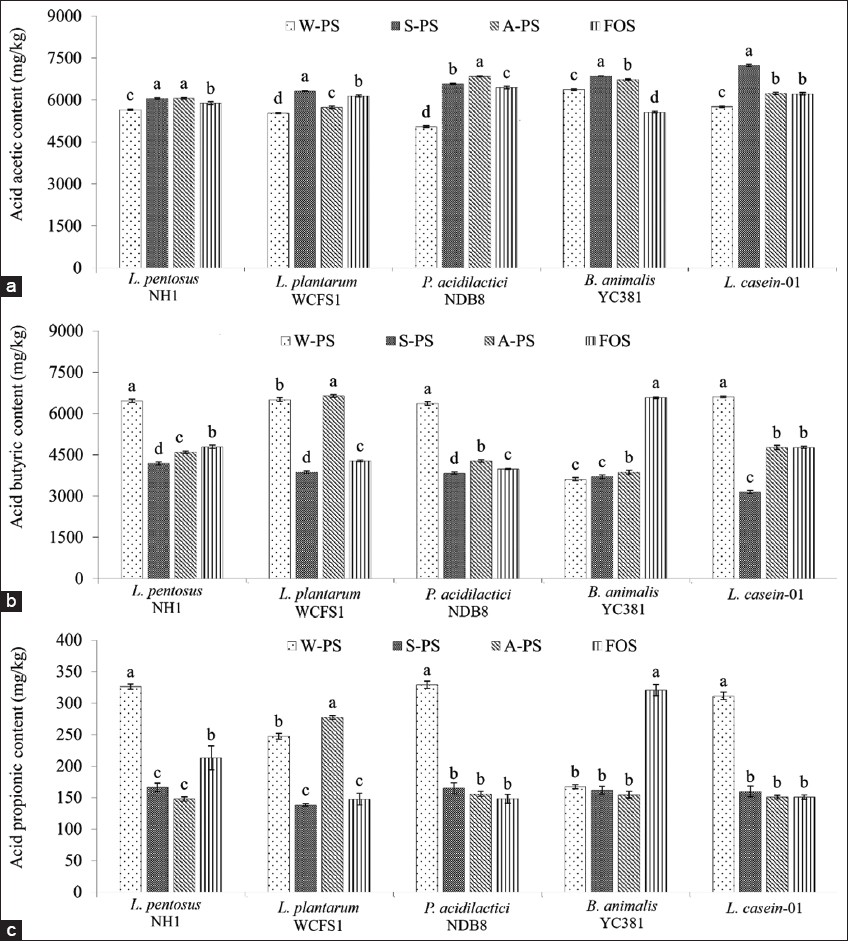 | Figure 6: Short-chain fatty acid content (mg/kg) after 24 h of fermentation. (a) Acetic acid, (b) Butyric acid, (c) Propionic acid. Different letters within the same strain indicate significant differences (Tukey’s Honestly Significant Difference, P < 0.05). [Click here to view] |
The type and structure of PSs significantly influence fermentation efficiency. Highly branched PSs may require specialized microbial enzymes, potentially leading to lower SCFA yields [57]. FOS, composed of simpler oligosaccharides, is more readily fermented, resulting in higher SCFA production. Furthermore, probiotic strains vary in their ability to produce SCFAs. Strains such as Lactobacillus rhamnosus, Bifidobacterium longum, and Bifidobacterium breve are known for their efficient PS fermentation and SCFA production. The presence of genes encoding appropriate enzymes is crucial for efficient PS utilization. This explains the observed variations in SCFA production among different strains and PS sources.
The diversity and composition of gut microbiota also play a significant role in SCFA production. Studies have shown that specific bacterial strains, such as Lactobacillus faecis, can modulate gut microbiota and influence SCFA content [58].
The cultivation of probiotics with PS supplementation significantly impacts SCFAs production, influencing gut health, immune function, and metabolic processes. The interaction between specific probiotic strains and PSs, such as inulin, resistant starch, and pectin, enhances the production of butyrate, acetate, and propionate. Optimizing the selection of probiotics and PSs can harness these beneficial effects for improved health outcomes. Further research into strain-specific interactions and their effects on SCFAs production will continue to advance probiotic-based therapies.
4. CONCLUSION
This study characterized three PS extracts, revealing distinct structural and functional properties influenced by both the extraction solvent and the resulting sequence. Notably, the water-extracted PS fraction (W-PS) demonstrated both the highest PS yield and the most potent ABTS free radical scavenging activity among the tested samples. PS extracts obtained from neutral and alkaline solvents demonstrated greater reducing power compared to those from acidic solvents. All PS extracts displayed prebiotic activity, selectively stimulating probiotic growth and exhibiting varying SCFA production capacities across different strains.
These findings highlight the significant impact of extraction methods and resulting PS sequences on biological activity. By strategically employing different extraction techniques, it is possible to optimize the recovery of PS fractions with targeted bioactivities from C. militaris mycelia. This approach opens up new avenues for developing functional foods, nutraceuticals, and pharmaceuticals to meet the growing demand for natural health-promoting products.
5. ACKNOWLEDGMENTS
We thank BS. Truong Cong Phat for assisting us in assessing and arranging facilities for tests at the Laboratory of Mushroom Technology, Faculty of Biology, Agriculture, and Environmental Sciences - University of Science and Education, The University of Danang.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. FUNDING
There is no funding to report.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
11. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
12. Use of artificial intelligence (AI)-assisted technology
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Zhang J, Wen C, Duan Y, Zhang H, Ma H. Advance in Cordyceps militaris (linn) link polysaccharides:Isolation, structure, and bioactivities:A review. Int J Biol Macromol. 2019;132:906-14.[CrossRef]
2. Krishna KV, Ulhas RS, Malaviya A. Bioactive compounds from cordyceps and their therapeutic potential. Crit Rev Biotechnol. 2024;44(5):753-73.[CrossRef]
3. Mironczuk-Chodakowska I, Kujawowicz K, Witkowska AM. Beta-glucans from fungi:Biological and health-promoting potential in the COVID-19 pandemic era. Nutrients. 2021;13:3960.[CrossRef]
4. Kanlayavattanakul M, Lourith N. Cordyceps militaris polysaccharides:Preparation and topical product application. Fungal Biol Biotechnol. 2023;10(1):3.[CrossRef]
5. Rupa EJ, Li JF, Arif MH, Yaxi H, Puja AM, Chan AJ, et al. Cordyceps militaris fungus extracts-mediated nanoemulsion for improvement antioxidant, antimicrobial, and anti-inflammatory activities. Molecules. 2020;25:5733.[CrossRef]
6. Li SP, Zhao KJ, Ji ZN, Song ZH, Dong TT, Lo CK, et al. A polysaccharide isolated from cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003;73(19):2503-13.[CrossRef]
7. Shashidhar GM, Giridhar P, Manohar B. Functional polysaccharides from medicinal mushroom Cordyceps sinensis as a potent food supplement:Extraction, characterization and therapeutic potentials –a systematic review. RSC Adv. 2015;5(21):16050-66.[CrossRef]
8. Chen X, Wu G, Huang Z. Structural analysis and antioxidant activities of polysaccharides from cultured Cordyceps militaris. Int J Biol Macromol. 2013;58:18-22.[CrossRef]
9. Chen J, Zou Y, Zheng T, Huang S, Guo L, Lin J, et al. The in vitro fermentation of Cordyceps militaris polysaccharides changed the simulated gut condition and influenced gut bacterial motility and translocation. J Agric Food Chem. 2022;70(44):14193-204.[CrossRef]
10. Ying M, Yu Q, Zheng B, Wang H, Wang J, Chen S, et al. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr Polym. 2020;235:115957.[CrossRef]
11. Lee BH, Chen CH, Hsu YY, Chuang PT, Shih MK, Hsu WH, et al. Polysaccharides obtained from Cordyceps militaris alleviate hyperglycemia by regulating gut microbiota in mice fed a high-fat/sucrose diet. Foods. 2021;10:1870.[CrossRef]
12. Sun L, Yuan H, Ma H, Wang Y. Effects of Cordyceps cicadaepolysaccharide on gut microbiota, the intestinal mucosal barrier, and inflammation in diabetic mice. Metabolites. 2025;15:8.[CrossRef]
13. Peng Z, Tian S, Li H, Zhu L, Zhao Z, Zheng G, et al. Extraction, characterization, and antioxidant properties of cell wall polysaccharides from the pericarp of Citrus reticulatacv. Chachiensis. Food Hydrocoll. 2023;136:108237.[CrossRef]
14. Yu D, Wang W, Hou S, Chang M, Chen Y, Meng J, et al. The effect of sequential extraction on the physicochemical and rheological properties of Naematelia aurantialba polysaccharides. Int J Biol Macromol. 2024;265:130777.[CrossRef]
15. Tian S, Peng Z, Zhang J, Yan D, Liang J, Zhao G, et al. Structural analysis and biological activity of cell wall polysaccharides and enzyme-extracted polysaccharides from pomelo (Citrus maxima (Burm.) Merr.). Int J Biol Macromol. 2024;279:135249.[CrossRef]
16. Nenadis N, Wang LF, Tsimidou M, Zhang HY. Estimation of scavenging activity of phenolic compounds using the ABTS (*+) assay. J Agric Food Chem. 2004;52(15):4669-74.[CrossRef]
17. Kosanic M, Rankovic B, Dašic M. Mushrooms as possible antioxidant and antimicrobial agents. Iran J Pharm Res. 2012;11(4):1095-102.
18. Phirom-On K, Apiraksakorn J. Development of cellulose-based prebiotic fiber from banana peel by enzymatic hydrolysis. Food Biosci. 2021;41:101083.[CrossRef]
19. Huebner J, Wehling RL, Hutkins RW. Functional activity of commercial prebiotics. Int Dairy J. 2007;17(7):770-5.[CrossRef]
20. Chen P, Lei S, Tong M, Chang Q, Zeng B, Zhang y, et al. Effect of polysaccharide fractions from Fortunella margarita on the fecal microbiota of mice and SCFA production in vitro. Food Sci Hum Wellness. 2022;11(1):97-108.[CrossRef]
21. Chen Y, Yao F, Ming K, Wang D, Hu Y, Liu J, et al. Polysaccharides from traditional Chinese medicines:Extraction, purification, modification, and biological activity. Molecules. 2016;21:1705.[CrossRef]
22. Liu Y, Wang J, Wang W, Zhang H, Zhang X, Han C, et al. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid Based Complement Alternat Med. 2015;2015(1):575063.[CrossRef]
23. Zhong S, Pan H, Fan L, Guoying LV, Wu Y, Parmeswaran B, et al. Advances in research of polysaccharides in Cordycepsspecies. Food Technol Biotechnol. 2008;47:304-12.
24. He BL, Zheng QW, Guo LQ, Huang JY, Yun F, Huang SS, et al. Structural characterization and immune-enhancing activity of a novel high-molecular-weight polysaccharide from Cordyceps militaris. Int J Biol Macromol. 2020;145:11-20.[CrossRef]
25. Xu L, Wang F, Zhang Z, Terry N. Optimization of polysaccharide production from Cordyceps militaris by solid-state fermentation on rice and its antioxidant activities. Foods. 2019;8:590.[CrossRef]
26. Miao M, Yu WQ, Li Y, Sun YL, Guo SD. Structural elucidation and activities of Cordyceps militaris-derived polysaccharides:A review. Front Nutr. 2022;9:898674.[CrossRef]
27. Dong Y, Hu S, Liu C, Meng Q, Song J, Lu J, et al. Purification of polysaccharides from Cordyceps militaris and their anti-hypoxic effect. Mol Med Rep. 2015;11(2):1312-17.[CrossRef]
28. Li JH, Zhu YY, Gu FT, Wu JY. Efficient isolation of immunostimulatory polysaccharides from Lentinula edodes by autoclaving-ultrasonication extraction and fractional precipitation. Int J Biol Macromol. 2023;237:124216.[CrossRef]
29. Gong P, Wang S, Liu M, Chen F, Yang W, Chang X, et al. Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides:A mini-review. Carbohydr Res. 2020;494:108037.[CrossRef]
30. Zheng Y, Zhang S, Wang Q, Lu X, Lin L, Tian Y, et al. Characterization and hypoglycemic activity of a b-pyran polysaccharides from bamboo shoot (Leleba oldhamiNakal) shells. Carbohydr Polym. 2016;144:438-46.[CrossRef]
31. Jing Y, Yan M, Zhang H, Liu D, Qiu X, Hu B, et al. Effects of extraction methods on the physicochemical properties and biological activities of polysaccharides from Polygonatum sibiricum. Foods. 2023;12:2088.[CrossRef]
32. Liu Z, Li H, Liu Q, Feng Y, Wu D, Zhang X, et al. Ultrasonic treatment enhances the antioxidant and immune-stimulatory properties of the polysaccharide from Sinopodophyllum hexandrumfruit. Foods. 2023;12(5):910.[CrossRef]
33. Hashemifesharaki R, Xanthakis E, Altintas Z, Guo Y, Gharibzahedi SM. Microwave-assisted extraction of polysaccharides from the marshmallow roots:Optimization, purification, structure, and bioactivity. Carbohydr Polym. 2020;240:116301.[CrossRef]
34. Hang LT, Thu TN, Bich PB. Effect of plant oils on mycelial biomass production, biosynthesis and antioxidants of exopolysaccharide by Cordyceps sinensis. J Sci Technol Food. 2017;13(1):3-10.
35. Sharma SK, Gautam N, Atri NS. Optimization, composition, and antioxidant activities of exo- and intracellular polysaccharides in submerged culture of Cordyceps gracilis (Grev.) Durieu and Mont. Evid Based Complement Alternat Med. 2015;2015(1):462864.[CrossRef]
36. Sharma SK, Gautam N, Atri NS. Optimized extraction, composition, antioxidant and antimicrobial activities of exo and intracellular polysaccharides from submerged culture of Cordyceps cicadae. BMC Complement Altern Med. 2015;15:446.[CrossRef]
37. Wang X, Zhang J, Zhang KG, Guo Z, Xu G, Huang L, et al. Ultrasound-assisted enzyme extraction, physicochemical properties and antioxidant activity of polysaccharides from Cordyceps militaris solid medium. Molecules. 2024;29:4560.[CrossRef]
38. Chen R, Jin C, Li H, Liu Z, Lu J, Li S, et al. Ultrahigh pressure extraction of polysaccharides from Cordyceps militaris and evaluation of antioxidant activity. Sep Purif Technol. 2014;134:90-9.[CrossRef]
39. Bai L, Xu D, Zhou YM, Zhang YB, Zhang H, Chen YB, et al. Antioxidant activities of natural polysaccharides and their derivatives for biomedical and medicinal applications. Antioxidants (Basel). 2022;11:2491.[CrossRef]
40. Kim HM, Song Y, Hyun GH, Long NP, Park JH, Hsieh YS, et al. Characterization and antioxidant activity determination of neutral and acidic polysaccharides from Panax ginseng C. A. Meyer. Molecules. 2020;25:791.[CrossRef]
41. Yan JK, Wang WQ, Ma HL, Wu JY. Sulfation and enhanced antioxidant capacity of an exopolysaccharide produced by the medicinal fungus Cordyceps sinensis. Molecules. 2013;18:167-77.[CrossRef]
42. Dhahri M, Sioud S, Alsuhaymi S, Almulhim F, Haneef A, Saoudi A, et al. Extraction, characterization, and antioxidant activity of polysaccharides from ajwa seed and flesh. Separations 2023;10:103.[CrossRef]
43. Wang JM, Sun XY, Ouyang JM. Structural characterization, antioxidant activity, and biomedical application of Astragalus polysaccharide degradation products. Int J Polym Sci. 2018;2018(1):5136185.[CrossRef]
44. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687-701.[CrossRef]
45. De Figueiredo FC, Ranke FF, De Oliva-Neto P. Evaluation of xylooligosaccharides and fructooligosaccharides on digestive enzymes hydrolysis and as a nutrient for different probiotics and SalmonellaTyphimurium.LWT.2020;118:108761.[CrossRef]
46. Song H, Zhang Z, Li Y, Zhang Y, Yang L, Wang S, et al. Effects of different enzyme extraction methods on the properties and prebiotic activity of soybean hull polysaccharides. Heliyon. 2022;8(11):11053.[CrossRef]
47. Yeung YK, Kang YR, So BR, Jung SK, Chang YH. Structural, antioxidant, prebiotic and anti-inflammatory properties of pectic oligosaccharides hydrolyzed from okra pectin by Fenton reaction. Food Hydrocoll. 2021;118:106779.[CrossRef]
48. Zou X, Cai J, Xiao J, Zhang M, Jia X, Dong L, et al. Purification, characterization and bioactivity of different molecular-weight fractions of polysaccharide extracted from Litchi pulp. Foods. 2023;12(1):194.[CrossRef]
49. Wang T, Ye Z, Liu S, Yang Y, Dong J, Wang K, et al. Effects of crude Sphallerocarpus gracilis polysaccharides as potential prebiotics on acidifying activity and growth of probiotics in fermented milk. LWT. 2021;149:111882.[CrossRef]
50. Synytsya A, MíckováK, Synytsya A, Jablonsky I, Spevacek A, Erban V, et al. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatusand Pleurotus eryngii:Structure and potential prebiotic activity. Carbohydr Polym. 2009;76(4):548-56.[CrossRef]
51. Palframan R, Gibson GR, Rastall RA. Development of a quantitative tool for the comparison of the prebiotic effect of dietary oligosaccharides. Lett Appl Microbiol. 2003;37(4):281-4.[CrossRef]
52. Barrangou R, Altermann E, Hutkins R, Cano R, Klaenhammer TR. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc Natl Acad Sci U S A. 2003;100:8957-62.[CrossRef]
53. Rattanakiat S, Pulbutr P, Khunawattanakul W, Sungthong B, Saramunee K. Prebiotic activity of polysaccharides extracted from Jerusalem artichoke tuber and development of prebiotic granules. Pharmacogn J. 2020;12:1402-11.[CrossRef]
54. Sargautiene V, Nakurte I, Nikolajeva V. Broad prebiotic potential of non-starch polysaccharides from oats (Avena sativa L.):An in vitro study. Pol J Microbiol. 2018;67(3):307-13.[CrossRef]
55. Islamova ZI, Ogai DK, Abramenko OI, Lim AL, Abduazimov BB, Malikova MK, et al. Comparative assessment of the prebiotic activity of some pectin polysaccharides. Pharm Chem J. 2017;51(4):288-91.[CrossRef]
56. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article:The role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104-19.[CrossRef]
57. Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S. Short chain fatty acids and its producing organisms:An overlooked therapy for IBD? EBioMedicine. 2021;66:103293.[CrossRef]
58. Yang M, Meng F, Gu W, Fu L, Zhang F, Li F, et al. Influence of polysaccharides from Polygonatum kingianum on short-chain fatty acid production and quorum sensing in Lactobacillus faecis. Front Microbiol. 2021;12:758870.[CrossRef]