1. INTRODUCTION
Approximately 20% of human cancers have been found to be associated with inflammation and connected to infection or infectious agents [1–3]. Various sexually transmitted infections (STIs), such as Herpes, gonorrhea, Trichomonas vaginalis, HPV, and EBV have been identified as contributors to both the initiation and progression of cancer [4–6]. Cancer, characterized by abnormal growth of cells, carries an increased risk influenced by multiple factors. Those factors include poor emotional and physical health, high-stress levels, environmental disparities, along with genetics, family history of cancer, and chronic inflammation due to various factors including STIs [7–10].
There has been evidence indicating that the presence of asymptomatic infection contributes to the development of cancers, primarily due to prolonged chronic inflammation [11,12]. This association has been observed to contribute to racial disparities in several cancers including lung, colorectal, liver, prostate, cervical, breast cancer, and so on [13–15]. Studies have demonstrated a significantly higher incidence of prostate cancer among men with a history of exposure to any sexually transmitted infections including gonorrhea, HPV and EBV [16–18]. For cervical cancer, the fourth most diagnosed cancer in female, HPV infection is one of the most important risk factors [19]. Additional evidence has revealed an association between HPV and EBV, which modify expression patterns in various cellular factors including inflammation, metastasis, and tumor progression, suggesting that coinfection contributes to human malignancies including cervical, nasopharyngeal, and prostate tumorigenesis [20–24]. HIV-1 virus has been linked to an increased risk of developing various types of cancer, primarily due to HIV-1-induced immune suppression [25–27]. For example research indicates that HIV-positive women face an elevated risk of contracting HPV infections and developing cervical cancer [28, 29].
These studies firmly assert that microbial infection plays a significant role in the susceptibility to carcinogenesis and cancer development. In resource-limited communities, infections account for nearly a quarter of all cancer cases, a significant and concerning statistic [30]. Rural communities face a disproportionately higher burden of STIs compared to their counterparts, particularly those with history of certain STIs, facing the higher incidence of cancers and are likely to have more advanced stage cancers compared to other groups [31–38]. Studies showed the prevalence of viral and bacterial infections such as T. vaginalis, HPV, gonorrhea as well as prostate inflammation, in African American men than in those of Caucasian men [2, 17, 18, 36–41]. Similarly, The association between HPV infection and cervical cancer is undeniable, with African American women being the most likely to die from cervical cancer among all women affected by this disease [42–45]. Evidence showed that chronic infections with these pathogens can lead to sustained inflammation, immune system modulation, and direct genetic damage, all of which can contribute to cancer development. This, in turn, plays a significant role in cancer stage at diagnosis and survival rates. These facts underscore the urgent necessity for the early detection of pathogenic triggers linked to cancer development, particularly within high-risk communities.
Presently, nucleic acid amplification-based detection methods such as conventional polymerase chain reaction/reverse transcription polymerase chain reaction (PCR/RT-PCR) are widely used and golden standard for diagnosis of infectious agents [46,47]. Although sensitive and reliable yet, the reliance on these methodologies involves expensive equipment, reagents, trained technicians, and sample transportation to the laboratories equipped with required instruments. This poses a significant challenge for rural, remote, and underserved communities, where access to laboratories and research infrastructure is often nonexistent. Consequently, there is an urgent need to develop a simple, single-step, ultrasensitive, and cost-effective DNA/RNA amplification-based assay technique, enabling the identification of potential pathogen sources swiftly, whether at the PON or out in the field. The isothermal nucleic acid amplification technique amplifies the DNA/RNA at a constant temperature thus eliminating the need of thermocycler [48]. Strand displacement amplification, nucleic acid sequence-based amplification, Helicase-dependent amplification, rolling circle amplification, multiple strand displacement amplification (MDA), transcription-mediated amplification, signal-mediated amplification of RNA technology, and loop-mediated isothermal amplification (LAMP) are some most frequently used isothermal nucleic acid amplification techniques use for the detection of the infectious agents [48–52]. All these techniques have diverse methods of amplification and detections; therefore, different requirements, strengths, and weaknesses depending on the need. LAMP is a simple, highly sensitive, and cost-effective, nucleic acid amplification technique [53–56]. Due to its capability to rapidly produce large quantities of DNA/RNA and allow naked-eye visualization of the amplified product, this technology has become widely utilized for molecular pathogen detection [52,57–60]. Unlike PCR, it does not require thermocycling and reactions take place at a constant temperature (60°C–65°C) using standard displacement reaction [46]. LAMP uses six different primers to bind six regions of a target sequence, making this technique highly specific for the target sequence and eliminating the problem of non-target amplification, and taking as little as 30 minutes [53,61]. WarmStart RTx Reverse Transcriptase (New England Biolabs, UK) has combined reverse transcription (RT) and LAMP together in a single reaction making it possible to amplify RNA in one step [47,61], shortening the reaction time and allowing the rapid detection of pathogens. In addition, this technique is very simple, and its simplicity makes it practical to be used in remote communities, private clinics, and in the field to detect pathogens. In this work, we developed and standardized a simple colorimetric one-step LAMP assay for rapid detection of HIV-1, HPV-16, and EBV and analyzed them for their specificity and limit of detection. We designed custom primers targeting conserved regions of the respective pathogenic RNA to ensure high specificity and minimize the risk of cross-reactivity. Viral RNA amplification can be confirmed by visualizing a simple color change by the naked eye within as short as 25 minutes. This method can serve as a powerful detection tool for low-intensity infection due to its sensitivity and selectivity and helps cancer risk reduction and management.
2. MATERIALS AND METHODS
2.1. Virus Source
EBV Verification panel, HIV-1 verification panel, and the HPV Genotype 16 Verification Panel were from Exact Diagnostics. The verification panels (EBV and HIV-1) contain the heat-inactivated whole virus. The HPV Genotype 16 Verification Panel is formulated from human cell lines containing an integrated HPV virus.
2.2. RNA Extraction
Total RNAs were extracted from virus sources using TRI Reagent Solution, according to the manufacturer’s protocol {TRI Reagent® Solution Protocol (PN 9738M Rev D)}. The elution volume for RNA extraction was 22 µl.
2.3. LAMP Primer Design
Four different sets of primers were designed for each pathogen using LAMP Designer 1.15 (Premier Biosoft Primer Explorer V5 software). Primer sets were selected based on the guidelines from “A Guide to LAMP Primer Design”. Each set of primers comprises the forward inner primer (FIP), backward inner primer (BIP), forward outer primer (F3), backward outer primer (B3), Loop-forward primer (LF), and loop-backward primer (LB). Designed primer sets targeted the conserved genes of their respective pathogens. All the primer sets were evaluated for their ability to amplify the target RNA by LAMP. One set of primers for each pathogen was selected and used for further studies (Table 1). Depicts the sequences of selected primers set for HIV-1, EBV, and HPV-16. Primers were named based on the region of the genome in which sequences were derived. Primer sequences were blasted using BLAST: Basic Local Alignment Search Tool (nih.gov) to ensure specificity to its target pathogen.
 | Table 1. List of selected set of primers for each pathogen used in this study. [Click here to view] |
2.4. One-Step LAMP Reaction
LAMP reactions were prepared to amplify the target pathogenic RNAs. A 10X primer mix for each primer set was prepared beforehand to be used for LAMP reactions. LAMP reactions were carried out using WarmStartTM Colorimetric LAMP 2X Master Mix from New England Biolabs. A 20 µl reaction mixture (LAMP 2X Master Mix, 10 µl; pathogen-specific primer mix, 2 µl; RNA target, 1 µl; DNase & RNase-free molecular grade water, 7 µl) was mixed and centrifuged for 1 s. LAMP was performed in a thermocycler at 65°C for 15–60 minutes. To ensure the reliability of this assay, negative control reactions were prepared by replacing the target RNA with ultrapure water. Amplification can be directly detected by observing the color change with the naked eye, and gel electrophoresis was performed to confirm the result. All the LAMP experiments were carried out with a minimum of three replicates.
2.5. Gel Electrophoresis
LAMP reaction products were analyzed by using gel electrophoresis to confirm the presence of amplicons. Five microliters of amplified products were run in 1.5% agarose gel containing ethidium bromide, by using 1X TBE buffer at 100V for 30 minutes. The presence of amplicons was confirmed by visualizing the gel under a UV transilluminator system.
2.6. Sensitivity and Specificity
The selected primers were tested for both sensitivity in terms of time and copy numbers, and for specificity. The time sensitivity was determined by performing reactions at time intervals of 15, 25, 30, 45, and 60 minutes. To determine the minimum copy number that can be identified by this assay, 10-fold serial dilutions of HIV-1, HPV-16, and EBV RNA templates ranging from 4 to 40,000 RNA copies per reaction were prepared. An aliquot of each dilution was used as an RNA template to perform the LAMP reaction for a total of 15–60 minutes. The specificity test was performed by using the RNA of non-target pathogens to determine that the assay specifically amplifies only their pathogen of interest. Each set of primers should only be able to amplify its target RNA; to test primer specificity we crossed each primer set with non-target RNA. In particular, HIV-1 and EBV RNAs were used as templates with HPV-L2 primer, while EBV and HPV-16 RNAs were used as templates with HIV-GAG primer, and HIV-1 and HPV-16 RNAs were used as templates with EBV-BGLF primer. The LAMP reactions were run for a total of 15–60 minutes, followed by gel electrophoresis.
3. RESULTS
3.1. Screening of LAMP Primers for Each Pathogen
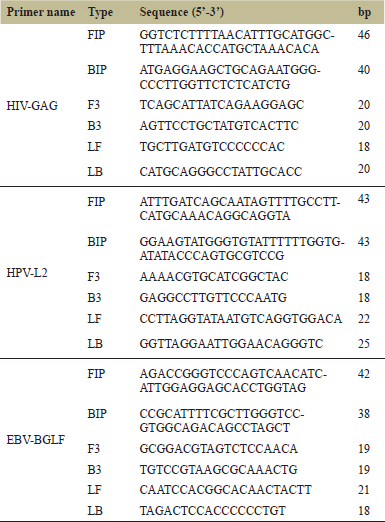
Four different sets of LAMP primers for HIV-1, HPV-16, and EBV targeting the conserved genes of each pathogenic RNA, respectively, were designed using Primer Explorer V5 software. Each set has six primers FIP, BIP, F3, B3, LF, and LB. To determine the ability of each set of primers to successfully amplify their target RNA, the LAMP reaction was carried out with the use of colorimetric pH-sensitive detection dye. Results demonstrated that HIV-GAG (Fig. 1A left panel), EBV-BGLF (Fig. 1B left panel), and HPV-L2 (Fig. 1C left panel) successfully amplified their target RNA by LAMP reaction. Each reaction was carried out with three replicates and results were consistent showcasing the efficacy of these set of primers in amplification of their target RNA. Therefore, HIV-GAG, EBV-BGLF, and HPV-L2 primer sets were selected as optimal primers for detecting HIV-1, EBV, and HPV-16 viruses, respectively, and used for further studies. No amplification was detected in any negative control sample using any primer set (Fig. 1 A–C, right panels). The color change was noted with the naked eyes, positive samples turned pink to yellow while negative samples with no template remained pink in color.
 | Figure 1. Pathogen specific LAMP primer screening. LAMP primers designed for HIV-1, EBV, and HPV-16 pathogens were screened to test sucessful amplification of their respective target pathogens. Pink color indicates no amplification and yellow color indicates sucessful amplification. A. HIV-GAG primer amplified target HIV-1 RNA. B. EBV-BGLF primer amplified target EBV RNA. C. HPV-L2 primer amplified target HPV-16 RNA. Negative Control (NC) for all three reactions stayed pink indicating no reaction. [Click here to view] |
3.2. Sensitivity of LAMP Assay
The selected LAMP primer sets were next tested to determine the lowest time limit of detection and the minimum copy number of RNA detectable under the reaction conditions described previously in section 2.6. To determine the earliest time point of detection, amplification reactions were carried out in triplicate at various time intervals from 15 to 60 minutes. As shown in Fig 2. A. all three primers HIV-GAG, HPV-L2, and EBV-BGLF primer were able to detect their respective RNAs within 25 minutes. The colorimetric result corresponds to the gel electrophoresis. Positive reactions with amplified product produced many bands of different sizes, similar to a typical ladder-like pattern on a 1.5% agarose gel, whereas no bands were observed from negative control reactions (Fig. 2B).
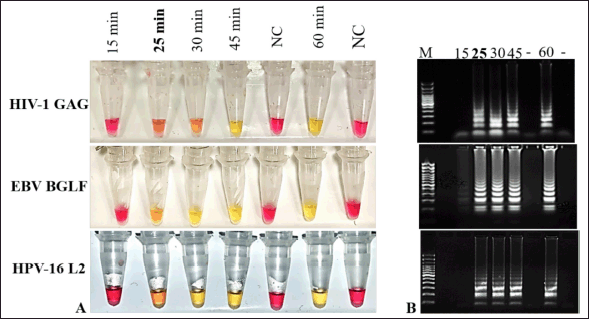 | Figure 2. Reaction time optimization for LAMP assay. A. To determine the time sensitivity of selected primers LAMP reactions were carried out at different time intervals from 15 to 60 minutes. The shortest time of detection. was recorded at 25minutes for all three HIV1-GAG, EBV-BGLF, and HPV-16 L2 primer sets. B. Gel electrophoresis results corresponded with colorimetric color detection results. Pink color reaction and absence of bands in gel of Negative control reactions (NC) indicate no reactivity. [Click here to view] |
The sensitivity of the assay in terms of RNA copy number was analyzed using 10-fold serial dilutions ranging from 40,000–4 RNA copies per reaction in triplicates. As shown in Figure 3A the detection limit for all three primers HIV-GAG, EBV-BGLF, and HPV-L2 was as low as 4 RNA copies per reaction. The colorimetric color change (Fig. 3A) readings were in complete accordance with the results of gel electrophoresis (Fig.3B).
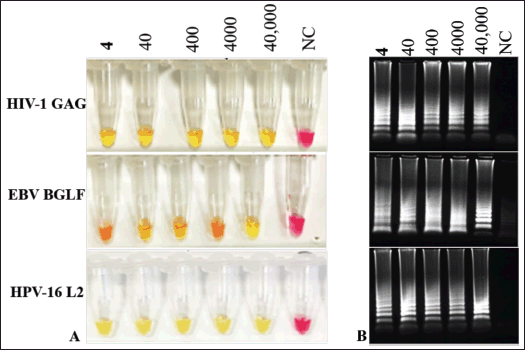 | Figure 3. Lowest limit of RNA copy number detection by LAMP. A. RNA detection limit of each primer set was examined by using 10-fold serial dilution of their respective pathogenic RNA ranging from 40,000 to 4 RNA copies per reaction. Each primer set was able detect as low as 4 RNA copy number reaction by visual color change to yellow. B. Results of gel electrophoresis correlate with colorimetric results. No reaction observed in negative control reactions (pink color, and no band in gel). [Click here to view] |
3.3. Specificity of Pathogen-Specific LAMP Primers
To evaluate the pathogenic specificities of each primer, cross reaction assays with non-target RNAs as templates were performed. HIV-GAG primer was cross tested with HPV-16 and EBV RNAs, EBV-BGLF primers were cross tested with HIV-1 and HPV-16 RNAs, and HPV-L2 primer was cross tested with HIV-1 and EBV RNAs. Results revealed that all three primers HIV-GAG (Fig. 4A, left panel), EBV-BGLF (Fig. 4B, left panel), and HPV-L2 (Fig. 4C, left panel) are highly specific and only produced the amplicons when reacted with their respective pathogenic RNA. No cross reactivity was observed with non-target RNA templates (Fig. 4A–C, left panel). Indicating that HIV- GAG, HPV- L2, and EBV- BGLF primers are specific only to HIV-1, HPV-16, and EBV sequences, respectively. The same results were observed with all triplicate and gel electrophoresis results corresponding with color changes (Fig. 4A–C, right panels).
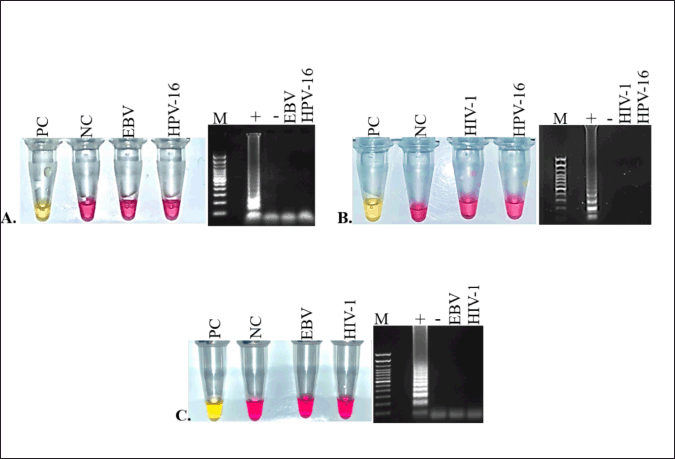 | Figure 4. Cross reactivity assay to determine the specificity of LAMP primers. Specificity of each primer set was tested by cross reacting the primer sets with non-target RNAs as template. Every positive control (PC) has primer and it’s respective target RNAs in reaction. Target RNAs were replaced by the distilled water in each negative control (NC) reaction. A. HIV-1 specific Gag primer cross-checked with EBV, and HPV-16 non-target RNAs. B. EBV specific BGLF primer cross-checked with HPV-16, and HIV-1 non-target RNAs. C. HPV 16 specific L2 primer cross-checked with EBP, and HIV1 non-target RNAs. No cross reactivity was observed with any primer set. Each primer set demonstrated 100% specificity to their respective target RNAs. Positive controls showed amplification (yellow color) and no reaction was observed in negative controls (pink color). [Click here to view] |
4. DISCUSSION
The early detection and timely identification of cancer-related pathogens such as HPV, EBV, and HIV can play a crucial role in understanding and managing illnesses, especially in asymptomatic patients. For rapid detection of viral infections directly from the source, PCR/RT-PCR and immunoassay methods are among the most popular approaches today [62]. PCR/RT-PCR is typically highly sensitive but can be expensive, time-consuming, and require costly thermocyclers and trained personnel to operate [63]. On the other hand, immunoassay-based detection is cheaper, more robust, and accessible to untrained users. However, antibody-based detection does not achieve the same level of sensitivity as nucleic acid amplification methods [63,64]. When choosing a method for pathogen detection, it is crucial to consider several factors, including whether the assay is intended for laboratory-based use or point-of-care (POC)/PON testing, the urgency of the diagnosis, and the associated costs. A POC test is conducted at or near the location where a patient first engages with the healthcare system. It offers rapid turnaround times and delivers reliable information that can be used to develop patient management plans. The immediacy of PON testing and the swift receipt of results reduce the need for multiple patient visits and enable timely treatment. In this study, a simple, rapid, and sensitive nucleic acid amplification-based assay was developed to detect three cancer-related pathogen HPV-16, EBV, and HIV-1 by using one-step LAMP colorimetric method. Four different sets of primers were designed for each pathogen and after screening HIV-GAG, EBV-BGLF, and HPV-L2 primer sets targeting HIV-1, EBV, and HPV-16 viruses, respectively, were selected. In consideration of the specificity and accuracy of pathogen detection, the assay was developed to specifically amplify a consered region of each pathogenic RNA and results were monitored by visual inspection of color change from pink to yellow. All positive samples identified by color change were also detected by gel electrophoresis. The result of gel electrophoresis was in complete accordance with visual color change detection. It eliminates the need of postamplification procedures like ethidium bromide staining and gel electrophoresis saving time, reducing the need of trained people, and making it suitable for PON use.
The LAMP assay exhibited a noteworthy amplification sensitivity both in terms of time and template copy number. All three pathogenic RNAs were amplified within 25 minutes by LAMP assay (Fig. 2). The LAMP assay successfully detected 4 RNA copies per reaction for all three pathogens HIV-1, EBV, and HPV-16 (Fig. 3) proving the high sensitivity of this method and making it possible to detect low concentration of pathogen present in the earlier stage of infections. Cross reactions with non-target RNAs were performed to further confirm the specificity of HIV-GAG, EBV-BGLF, and HPV-L2 primer sets. No false positive reactions were observed and none of the primers showed any cross reactivity with non-target RNAs (Fig. 4). These results demonstrated that all three primers showed 100% specificity and only amplified their target RNA. The 100% specificity of these primers presents a promising opportunity to develop a multiplex detection assay based on LAMP. This assay would enable the simultaneous detection of all three target pathogens by introducing distinct sets of primers to amplify specific DNA/RNA regions within a single reaction. We have developed a test for three cancer-related pathogens, highlighting the promising potential of the LAMP assay in clinically diagnosing various cancer-associated pathogens. Nonetheless, the reliability of this assay warrants additional scrutiny through comprehensive testing using a large-scale clinical sample pool. LAMP can be designed for multiplexing, enabling the simultaneous detection of multiple pathogens. The comparison of pathogen detection methods highlights the distinct advantages and limitations of each approach. Most used traditional PCR/Real-time PCR requires 2–4 hours to deliver results, incurs a cost of approximately $5–$10 per reaction, and requires an expensive thermocycler ($3,000–$25,000). Antigen–antibody detection methods, such as ELISA or lateral flow assays, provide results from 15 minutes to 2 hours at a lower cost of $1–$5 per reaction. However, these methods often lack the sensitivity required to detect low pathogen concentrations. In contrast, the LAMP assay is highly sensitive, and offers a rapid result within 25–30 minutes at a cost of $1–$2 per reaction, making it particularly suitable for PON testing and resource-limited settings. LAMP could serve as a powerful detection tool for low-intensity infection due to its sensitivity and selectivity and help cancer risk management and STI treatment decisions. Rural communities would benefit from this highly sensitive diagnostic to inform the timely treatment of infectious or even chronic diseases.
5. CONCLUSION
In conclusion, this study successfully developed a one-step colorimetric LAMP assay for the rapid and precise detection of HIV-1, HPV-16, and EBV. The assay exhibits outstanding sensitivity, enabling the identification of even minimal traces of these pathogens. Furthermore, it holds great potential for simultaneous and multiplexed detection of multiple human pathogens in a high-throughput format, ensuring highly accurate results even at very low pathogen concentrations.
6. ACKNOWLEDGMENTS
This research project was supported by the RCMI PILOT project under Parent Grant 5U54MD007585-27 and NSF/DBI/2039369 and the authors are very thankful for their funding support.
7. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
11. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
12. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 2006;124:823–35. CrossRef
2. Munn LL. Cancer and inflammation. WIREs Syst Biol Med 2017;9:e1370. CrossRef
3. Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep 2012;32:1–15. CrossRef
4. Nahand JS, Khanaliha K, Mirzaei H, Moghoofei M, Baghi HB, Esghaei M, et al. Possible role of HPV/EBV coinfection in anoikis resistance and development in prostate cancer. BMC Cancer 2021;21:926. CrossRef
5. Plummer M, Martel C de, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016;4:e609–16. CrossRef
6. Sokefun O, Akinnola O. Cancer-inducing mechanisms of representative sexually-transmitted infection pathogens. Cell Biol 2020;8:12–21. CrossRef
7. Hassanpour SH, Dehghani M. Review of cancer from perspective of molecular. J Cancer Res Pract 2017;4:127–9. CrossRef
8. Soto AM, Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat Rev Endocrinol 2010;6:363–70. CrossRef
9. Turati F, Bosetti C, Polesel J, Serraino D, Montella M, Libra M, et al. Family history of cancer and the risk of bladder cancer: a case-control study from Italy. Cancer Epidemiol 2017;48:29–35. CrossRef
10. Vineis P, Wild CP. Global cancer patterns: causes and prevention. Lancet 2014;383:549–57. CrossRef
11. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 2019;51:27–41. CrossRef
12. Rook GAW, Dalgleish A. Infection, immunoregulation, and cancer. Immunol Rev 2011;240:141–59. CrossRef
13. Ashktorab H, Kupfer SS, Brim H, Carethers JM. Racial disparity in gastrointestinal cancer risk. Gastroenterology 2017;153:910–23. CrossRef
14. DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, et al. Cancer statistics for African Americans. Progress and opportunities in reducing racial disparities. CA Cancer J Clin 2016;66:290–308. CrossRef
15. O’Keefe EB, Meltzer JP, Bethea TN. 2015. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front Public Health 2015;3:51. CrossRef
16. Mahmoudi S, Jafari-Sales A, Nasiri R, Baghi HB. Prostate cancer and human papillomavirus infection: a recent literature review. Rev Res Med Microbiol 2022;33:100. CrossRef
17. Perletti G, Monti E, Magri V, Cai T, Cleves A, Trinchieri A, Montanari E. The association between prostatitis and prostate cancer. Systematic review and meta-analysis. Arch Ital Urol Androl 2017;89:259–65. CrossRef
18. Taylor M, Mainous A, Wells B. Prostate cancer and sexually transmitted diseases: a meta-analysis. Fam Med 2004;37:506–12.
19. Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–19. CrossRef
20. Blanco R, Carrillo-Beltrán D, Osorio JC, Calaf GM, Aguayo F. Role of Epstein-barr virus and human papillomavirus coinfection in cervical cancer: epidemiology, mechanisms and perspectives. Pathogens 2020;9:685. CrossRef
21. Shi Y, Peng SL, Yang LF, Chen X, Tao YG, Cao Y. Co-infection of Epstein-barr virus and human papillomavirus in human tumorigenesis. Chin J Cancer 2016;35:16. CrossRef
22. Whitaker NJ, Glenn WK, Sahrudin A, Orde MM, Delprado W, Lawson JS. Human papillomavirus and Epstein barr virus in prostate cancer: koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer. Prostate 2013;73:236–41. CrossRef
23. Rickinson AB. Co-infections, inflammation and oncogenesis: future directions for EBV research. Semin Cancer Biol 2014;26:99–115. CrossRef
24. Feng M, Duan R, Gao Y, Zhang H, Qiao Y, Li Q, et al. Role of Epstein-barr virus and human papillomavirus coinfection in cervical intraepithelial neoplasia in Chinese women living with HIV. Front Cell Infect Microbiol 2021;11:703259. CrossRef
25. Loarca L, Fraietta JA, Pirrone V, Szep Z, Wigdahl BHuman immunodeficiency virus (HIV) infection and cancer. HIV/AIDS—Contemp Chall 2017;33:237−62; doi: https://doi.org/10.5772/67162 CrossRef
26. Shmakova A, Germini D, Vassetzky Y. HIV-1, HAART and cancer: a complex relationship. Int J Cancer 2020;146:2666–79. CrossRef
27. Silverberg MJ, Chao C, Leyden WA, Xu L, Horberg MA, Klein D, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev 2011;20:2551–9. CrossRef
28. Akbari E, Milani A, Seyedinkhorasani M, Bolhassani A. HPV co-infections with other pathogens in cancer development: a comprehensive review. J Med Virol 2023;95:e29236. CrossRef
29. Liu G, Sharma M, Tan N, Barnabas R. HIV-positive women have higher risk of HPV infection, precancerous lesions, and cervical cancer: a systematic review and meta-analysis. AIDS 2018;32:1. CrossRef
30. Gulley ML, Morgan DR. Molecular oncology testing in resource-limited settings. J Mol Diagn 2014;16:601–11. CrossRef
31. Newman LM, Berman SM. Epidemiology of STD disparities in African American communities. Sex Transm Dis 2008;35:S4. CrossRef
32. Dutta A, Uno H, Holman A, Lorenz DR, Gabuzda D. Racial differences in prostate cancer risk in young HIV-positive and HIV-negative men: a prospective cohort study. Cancer Causes Control 2017;28:767–77. CrossRef
33. Fernández L, Galán Y, Jiménez R, Gutiérrez Á, Guerra M, Pereda C, et al. Sexual behaviour, history of sexually transmitted diseases, and the risk of prostate cancer: a case–control study in Cuba. Int J Epidemiol 2005;34:193–7. CrossRef
34. Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis 2005;26:1170–81. CrossRef
35. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to Neighborhood. Cold Spring Harb Perspect Med 2018;8:a030387. CrossRef
36. Sarma Aruna V, McLaughlin Julie C, Wallner Lauren P, Dunn Rodney L, Cooney Kathleen A, Schottenfeld David, et al. Sexual behavior, sexually transmitted diseases and prostatitis: the risk of prostate cancer in black men. J Urol 2006;176:1108–13. CrossRef
37. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep 2017;18:81. CrossRef
38. Wallner LP, Clemens JQ, Sarma AV. Prevalence and risk factors for prostatitis in African American men: findings from the flint men’s health study. Prostate 2009;69:24–32. CrossRef
39. Massari F, Mollica V, Di Nunno V, Gatto L, Santoni M, Scarpelli M, et al. The human microbiota and prostate cancer: friend or foe? Cancers 2019;11:459. CrossRef
40. Miller WC, Swygard H, Hobbs MM, Ford CA, Handcock MS, Morris M, et al. The prevalence of trichomoniasis in young adults in the United States. Sex Transm Dis 2005;32:593. CrossRef
41. Moghoofei M, Keshavarz M, Ghorbani S, Babaei F, Nahand JS, Tavakoli A, et al. Association between human papillomavirus infection and prostate cancer: a global systematic review and meta-analysis. Asia-Pac J Clin Oncol 2019;15:e59–e67. CrossRef
42. Adegboyega A, Obielodan O, Wiggins AT, Dignan M, Williams LB. Beliefs and knowledge related to human papillomavirus (HPV) vaccine among African Americans and African immigrants young adults. Cancer Causes Control 2023;34:479–89. CrossRef
43. Gopalani SV, Janitz AE, Campbell JE. 2020. Cervical cancer incidence and mortality among Non-Hispanic African American and White Women, United States, 1999–2015. J Natl Med Assoc 2020;112:632–8. CrossRef
44. Ojeaga A, Alema-Mensah E, Rivers D, Azonobi I, Rivers B. Racial disparities in HPV-related knowledge, attitudes, and beliefs among African American and white women in the U.S. J Cancer Educ 2019;34:66–72. CrossRef
45. Strohl AE, Mendoza G, Ghant MS, Cameron KA, Simon MA, Schink JC, et al. Barriers to prevention: knowledge of HPV, cervical cancer, and HPV vaccinations among African American women. Am J Obstetr Gynecol 2015;212:65.e1–5. CrossRef
46. Parida M, Sannarangaiah S, Dash PK, Rao PVL, Morita K. Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol. 2008;18:407–21. CrossRef
47. Wong YP, Othman S, Lau YL, Radu S, Chee HY. Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms. J Appl Microbiol 2018;124:626–43. CrossRef
48. Srivastava P, Prasad D. Isothermal nucleic acid amplification and its uses in modern diagnostic technologies. 3 Biotech 2023;13:200. CrossRef
49. Boonbanjong P, Treerattrakoon K, Waiwinya W, Pitikultham P, Japrung D. Isothermal amplification technology for disease diagnosis. Biosensors 2022;12:677. CrossRef
50. Cao S, Tang X, Chen T, Chen G. Types and applications of nicking enzyme-combined isothermal amplification. Int J Mol Sci 2022;23:4620. CrossRef
51. Moon YJ, Lee SY, Oh SW. A review of isothermal amplification methods and food-origin inhibitors against detecting food-borne pathogens. Foods 2022;11:322. CrossRef
52. Motamedi MK, Saghafinia M, Karami A, Gill P. A review of the current isothermal amplification techniques: applications, advantages and disadvantages. J Global Infect Dis 2011;3:293. CrossRef
53. Lin Q, Ye X, Yang B, Fang X, Chen H, Weng W, et al. Real-time fluorescence loop-mediated isothermal amplification assay for rapid and sensitive detection of Streptococcus gallolyticus subsp. gallolyticus associated with colorectal cancer. Anal Bioanal Chem 2019;411:6877–87. CrossRef
54. Notomi T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 2000;28:63e–63. CrossRef
55. Sano S, Fukushi S, Yamada S, Harada S, Kinoshita H, Sugimoto S, et al. Development of an RT-LAMP assay for the rapid detection of SFTS virus. Viruses 2021;13:693. CrossRef
56. Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protocols 2008;3:877–82. CrossRef
57. Daskou M, Tsakogiannis D, Dimitriou TG, Amoutzias GD, Mossialos D, Kottaridi C, et al. WarmStart colorimetric LAMP for the specific and rapid detection of HPV16 and HPV18 DNA. J Virol Methods 2019;270:87–94. CrossRef
58. Feng W, Hieno A, Kusunoki M, Suga H, Kageyama K. LAMP detection of four plant-pathogenic oomycetes and its application in lettuce fields. Plant Dis 2019;103:298–307. CrossRef
59. Iwamoto T, Sonobe T, Hayashi K. Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in Sputum samples. J Clin Microbiol 2003;41:2616–22. CrossRef
60. Njiru ZK, Mikosza ASJ, Armstrong T, Enyaru JC, Ndung’u JM, Thompson ARC. Loop-mediated isothermal amplification (LAMP) method for rapid detection of Trypanosoma brucei rhodesiense. PLoS Negl Trop Dis 2008;2:e147. CrossRef
61. Huang WE, Lim B, Hsu CC, Xiong D, Wu W, Yu Y, et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb Biotechnol 2020;13:950–61. CrossRef
62. Krokhine S, Torabi H, Doostmohammadi A, Rezai P. Conventional and microfluidic methods for airborne virus isolation and detection. Colloids Surf Biointerfaces 2021;206:111962. CrossRef
63. Cassedy A, Parle-McDermott A, O’Kennedy R. Virus detection: a review of the current and emerging molecular and immunological methods. Front Mol Biosci 2021;8:637559. CrossRef
64. Benzigar MR, Bhattacharjee R, Baharfar M, Liu G. Current methods for diagnosis of human coronaviruses: pros and cons. Anal Bioanal Chem 2021;413:2311–30. CrossRef