1. INTRODUCTION
Rice (Oryza sativa L.) is a monocotyledonous annual grass belonging to the family Poaceae (Gramineae). It is characterized by a terminal panicle inflorescence with branched spikelets that develop into caryopsis fruits (grains) after fertilization [1]. As one of the world’s most important staple crops feeding more than half of the global population, rice cultivation and productivity are increasingly threatened by environmental challenges. Global temperatures are rising annually due to climate change, adversely affecting the productivity of major crops, including rice. Heat stress, defined as temperature exceeding the optimal range for plant growth, triggers a cascade of physiological and biochemical disruptions in plants [2]. At the cellular level, high temperatures cause protein denaturation, membrane damage, and increased reactive oxygen species (ROS) production, leading to oxidative stress. Photosynthesis is particularly vulnerable, with heat stress reducing chlorophyll content, disrupting photosystem II functionality, and inhibiting RuBisCO activity [1]. Heat stress also impairs pollen viability and fertilization, directly affecting reproductive success. In rice specifically, exposure to high temperatures affects various developmental stages, with flowering being the most sensitive-temperatures above 35°C during anthesis can cause spikelet sterility exceeding 80% [3]. These physiological disruptions collectively contribute to substantial yield losses, with global estimates suggesting rice yield reductions of 41% by the end of the century if adaptation strategies are not implemented [4]. Projections suggest that rice yields could decline by up to 10% for each degree Celsius increase in global mean temperature [5]. Heat stress not only impedes development processes, reduces growth and grain yield but also significantly impacts grain quality by reducing rice panicle size, milling characteristics, and amylose content [6]. Consequently, developing heat-tolerant rice varieties has become a crucial research strategy for ensuring global food security. BT7 (2n = 24), a high-quality indica rice variety widely cultivated in Northern Vietnam, stands out among other rice varieties for its superior grain quality, featuring clear grains, soft texture, and fragrant aroma [7,8]. However, BT7’s major drawback is its poor resilience to environmental stressors, particularly heat, which can significantly reduce yields. As a result, BT7 has consistently been a primary focus of rice improvement programs in Vietnam, aiming to enhance its heat tolerance while preserving its desirable qualities. To develop heat-tolerant rice varieties like BT7, it is essential to understand and manipulate the biochemical mechanisms that confer thermotolerance, with proline metabolism emerging as a promising target for improvement.
Proline (Pro) is recognized as one of the most effective osmoprotectants and signaling molecules in plant responses to various abiotic stresses, particularly in rice. It accumulates in the cytoplasm and contributes to cellular physiological adaptations, functioning as an osmolyte, antioxidant, metal chelator, protein stabilizer, ROS scavenger, and inhibitor of programmed cell death [9,10]. Proline synthesis involves key enzymes such as delta-1-pyrroline-5-carboxylate synthase (P5CS), pyrroline-5-carboxylate reductase (P5CR), and ornithine aminotransferase, while its catabolism is primarily mediated by proline dehydrogenase (ProDH). Under stress conditions, including heat stress, proline content increases through enhanced P5CS expression [11] or decreased ProDH expression [11,12]. While P5CS has been extensively studied [13,14], few investigations have directly examined ProDH’s role in stress tolerance responses, with most research focusing on the model plant Arabidopsis thaliana [11,15,16]. In the japonica rice variety Kongyu131 (KY131), OsProDH was shown to participate in thermotolerance response by regulating proline metabolism and ROS scavenging [12]. Notably, OsProDH expression under heat stress conditions can vary significantly among different rice varieties [11], suggesting a complex and genotype-specific role in heat tolerance mechanisms. Given its potential role in modulating proline levels under stress, OsProDH presents an intriguing target for enhancing heat tolerance in rice. While the role of OsProDH in rice heat tolerance shows promise, precisely modifying this gene requires advanced molecular tools that allow for targeted genetic manipulation without introducing foreign DNA.
CRISPR/Cas9 gene editing technology has emerged as a powerful tool in scientific research, particularly in plant sciences, due to its high efficiency and versatility in both basic and applied research [17]. This RNA-guided genome editing system functions through a simple mechanism where a guide RNA (gRNA) recognizes a specific complementary target DNA sequence, directing the Cas9 endonuclease to introduce a double-stranded break (DSB) at the target site. The break is then repaired either via non-homologous end joining, which often creates small insertions or deletions, or through homology-directed repair when a repair template is available. In rice, this system has been successfully applied to create precise mutations in various genes, with high efficiency and specificity, enabling researchers to study gene function and develop improved varieties [17]. The precision and efficiency of CRISPR/Cas9 make it ideally suited for investigating specific genes like OsProDH that regulate stress response pathways in economically important crops. In this study, we employed CRISPR/Cas9 to elucidate the functional role of OsProDH in the heat tolerance response of BT7. We chose to focus on OsProDH in BT7 due to the variety’s economic importance and its current susceptibility to heat stress. By precisely modifying the OsProDH gene, we aim to not only confirm its involvement in heat stress adaptation but also lay the groundwork for developing heat-tolerant BT7 rice varieties for commercial production. This approach demonstrates the potential of targeted gene editing in enhancing crop resilience to climate change-induced stresses while maintaining desirable agronomic traits.
2. MATERIALS AND METHODS
2.1. Plant Material and Growth Condition
Wild-type (WT) rice cultivar Bacthom 7 (O. sativa L. ssp. indica) was provided by Thaibinh Seed Corporation (Vietnam). Germinated seeds were placed into 96-well plates and grown in MS solution under controlled conditions of 16 h light/8 h dark cycle at 28°C ± 2°C with 70% relative humidity. For heat tolerance analysis, 2-week-old plants were subjected to heat stress in a constant climate chamber at 45°C for varying durations: 3 h for measurement of O2− radical accumulation and antioxidant enzyme activity, 9 h for measurement of malondialdehyde (MDA) content, chlorophyll content and relative water content (RWC), and 12 h for measurement of proline content and survival ratio. For screening and assessment of transgenic/edited plants, rice plants were cultivated in a net house under the following conditions: 30°C ± 2°C for 14 h (light) and 26°C ± 2°C for 10 h (dark) with 80% relative humidity.
2.2. Targeted Mutagenesis of OsProDH
Two gRNA sequences targeting Exon II of OsProDH [Figure 1a and b] were designed based on the sequencing result of the target gene using CRISPR-P v2.0 [13] and CCTop [18] tools. Off-target sequences were predicted for the OsProDH sgRNAs against the rice Nipponbare genome using default parameters [Supplemental Table 3]. The gRNA sequences were synthesized and cloned into the BtgZI and BsaI sites on the vector pENTR4-sgRNA as previously described [19]. Both sgRNA expression cassettes were then integrated into the plant transformation vector pCas9 [19] using Gateway LR clonase (Life Technologies). The resulting construct was introduced into Agrobacterium tumefaciens EHA105 for rice transformation using a previously established method [7].
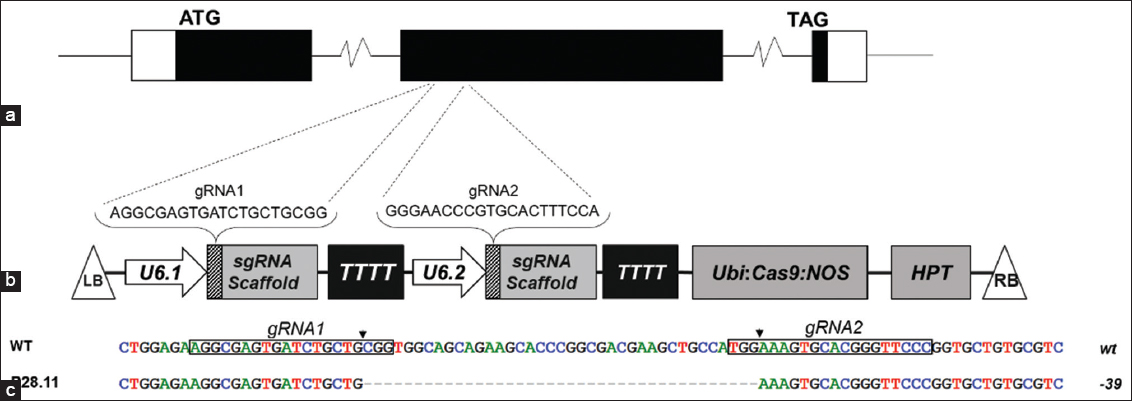 | Figure 1: CRISPR/Cas9-induced OsProDH mutation in BT7 rice. (A) Schematic diagram of OsProDH gene structure showing two target sites on exon II. Black rectangles: exons; lines: introns; white rectangles: UTRs. (B) T-DNA region with OsProDH gRNAs and key components: ZmUbiquitin promoter (Ubi); OsU6 promoters (U6.1, U6.2); Hygromycin resistance cassette (HPT); terminators (TTTT); left and right borders (LB, RB). (C) Alignment of edited OsProDH in T0 transgenic BT7 line P28 and wild-type (wt) OsProDH in non-edited (WT) plant, showing CRISPR/Cas9 cutting site (arrow), gRNA sites (box), and mutation (right labels). [Click here to view] |
The presence of the transgene in regenerated T0 plants was assessed through polymerase chain reaction (PCR) with HPT-specific primers (5’-AAACTGTGATGGACGACACCGT-3’ and 5′-GTGGCGATCCTGCAAGCTCC-3′). T1 individuals were screened by PCR using specific primer pairs for Cas9 (5′-ATGGCCCCAAAGAAGAAG-3′ and 5′-GCCTCGGCTGTCTCGCCA-3′), and HPT to select T-DNA-free lines. A DNA fragment containing gRNA sites was amplified with specific primers (5′-CGTGAAGGACTAGGGGATGG-3′ and 5′-TGCTCTGCTCCAACTCATCA-3′) from all transgenic T0 or transgene-free T1 plants for Sanger sequencing. OsProDH mutations were identified via decoding analysis of the sequencing chromatograms using the Degenerate Sequence Decoding method [20].
2.3. Physiological Parameters Measurement
For measurement of RWC, leaves were collected from treated plants and their fresh weight (FW), turgid weight, and dry weight were measured. For the survival ratio experiment, 2-week-old plants were subjected to 45°C treatment and then returned to normal conditions. The survival rate was observed after a 2-week recovery period, with plants showing regrowth and continued development considered as survivors.
2.4. Biochemical Parameters Assessment
Leaf samples from heat-stressed and control rice plants were homogenized and extracted as per the methods described previously [12,21,22]. Chlorophyll, MDA, proline, and O2− radical contents were determined spectrophotometrically. Absorbance was measured at 663/645 nm for chlorophyll, 532/600 nm for MDA, 520 nm for proline (using a 0–25 μg/mL L-proline standard curve), and 560 nm for O2− radicals. Each experiment was repeated 3 times with three biological replicates.
For enzyme assays, leaves were ground in liquid nitrogen with phosphate buffer. Catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX) activities were measured as described [23]. CAT activity was determined by H2O2 decomposition rate at 240 nm, POD by guaiacol oxidation at 470 nm, and APX by ascorbate oxidation at 290 nm. Enzyme activity was expressed as U/mg FW.
2.5. Agronomic Parameter Assessment
WT and selected mutant BT7 rice plants were cultivated in a net house under controlled environmental conditions. The experiment followed a randomized complete block design with three replications. At plant maturity, five individuals from each genotype per replication were evaluated for key agronomic traits. These traits included days to maturity, plant height, number of tillers per plant, number of filled grains per panicle, grain yield per plant, and amylose content. In total, 15 plants per genotype (5 plants × 3 replications) were assessed to ensure statistical robustness.
3. RESULTS
3.1. Generation of BT7 OsProDH Edited Lines via CRISPR/Cas9 Method
To investigate the role of OsProDH in heat stress response in BT7 rice, we generated OsProDH mutant BT7 rice lines. Two gRNA sequences targeting positions 39 bp apart in exon II of OsProDH [Figure 1a] were designed and cloned into a CRISPR/Cas9 rice transformation vector using two sgRNA cassettes driven by OsU6 promoters [Figure 1b]. The generated T-DNA constructs were verified by PCR with specific primer pairs [Supplemental Figure 1] and nucleotide sequencing [Supplemental Figure 2], then transformed into BT7 rice calli via Agrobacterium to induce mutations [Figure 2a-e].
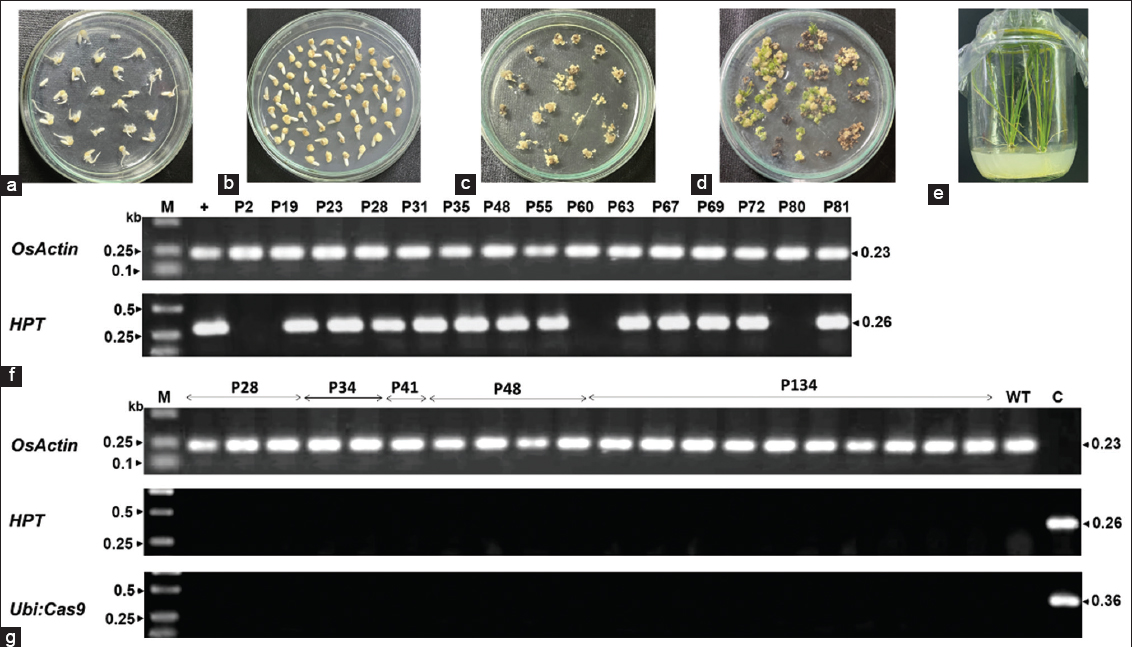 | Figure 2: BT7 rice transformation via A. tumefaciens. (A) Seven-day-old calli induced from BT7 mature embryos. (B) Co-cultivation of BT7 calli with A. tumefaciens carrying pCas9/sgRNA-OsProDH. (C) Selection of hygromycin-resistant calli. (D) Shoots developed from surviving calli after 40 days on regeneration medium. (E) Root generation from shooting calli. (F, G) PCR analysis of transgene integration in T0 (F) and T1 (G) generations using specific primer pairs for OsActin (endogenous control), HPT (marker gene) and Ubiquitin-Cas9 expression cassette. +: positive control; C: pCas9/sgRNA-OsProDH; WT: wild-type plant; M: 1.0 kb DNA ladder. [Click here to view] |
We identified 73 regenerated plants as T0 transgenic events through PCR analysis using HPT-specific primers [Figure 2f, Supplemental Table 1]. Sanger sequencing of 20 transgenic plants revealed OsProDH mutations in 17 plants [Supplemental Table 1] at both target sites, including deletions, insertions, and substitutions [Table 1]. Notably, the majority of mutations (23/26 mutant alleles) were DNA deletions (39–41 nucleotides) located between the two theoretical DSB sites [Supplemental Figure 3], indicating similar efficiency of the two gRNAs. The mutation genotypes observed were 40% homozygous, 25% heterozygous, and 20% biallelic OsProDH mutants [Table 1].
Table 1: Frequency of OsProDH mutation in T0 transgenic plants.
| Location | Mutant genotype ratiosa | Mutant type ratiosb | |||||
|---|---|---|---|---|---|---|---|
| Heterozygous | Homozygous | Bialellic | wt | Deletion | Insertion | Substitution | |
| gRNA1 | 8/20 | 5/20 | 4/20 | - | 23/26 | 2/40 | 1/40 |
| gRNA2 | 8/20 | 5/20 | 4/20 | - | 24/40 | 1/40 | 0/40 |
| gRNA1-gRNA2 | 8/20 | 5/20 | 4/20 | 3/20 | 24/40 | 1/40 | 1/40 |
a Number of genotypes/total number of genotypes.
b Number of allele mutation type/total number of all alleles.
To obtain homozygous mutant BT7 lines without T-DNA, we randomly selected five lines with different mutant genotypes (P28, P34, P41, P48, P134) for genotype screening of the T1 population. The results showed that the T-DNA structure segregated in the T1 population according to Mendel’s law (either χ²3:1 or χ²15:1 < 3.841) [Supplemental Table 2], and CRISPR/Cas9-induced mutations were stably inherited to the next generation [Figure 2g]. Among the T1 plants examined, three (P28.11, P28.31, P28.47) derived from line P28 simultaneously carried no T-DNA and had a homozygous mutant OsProDH genotype with a 39 bp deletion [Supplemental Table 2]. This mutation produced a functional protein with a deletion of 13 amino acids in the ProDH domain (data not shown).
A comprehensive assessment of the predicted off-target sites for our designed CRISPR/Cas9 system [Supplemental Table 3] revealed that they did not localize on or near any functional gene. Therefore, T2 seeds derived from P28.11 were randomly chosen for further study.
3.2. OsProDH Mutation Improves Proline Accumulation and Heat Tolerance of BT7 Edited Plants
To elucidate the role of OsProDH mutation in proline accumulation and physiological responses under heat stress, T2 plants of P28.11 and WT BT7 plants were subjected to artificial heat stress. The edited rice lines exhibited significantly higher survival and recovery rates compared to the control lines, both after long-term heat stress treatment of 2-month-old rice plants [Figure 3a and c] and short-term heat stress at the 2-week-old stage [Figure 3b and d]. Further analysis revealed that 2-week-old edited plants demonstrated superior water retention [Figure 3e] and photosynthetic protection [Figure 3f] compared to WT plants under heat stress conditions
 | Figure 3: Heat-resistant phenotype of OsProDH mutant. (a and b) Representative images of 2-month-old (a) and 2-week-old (b) P28.11 plants after 2 weeks of recovery from heat stress. (c and d) Survival rates of 2-month-old (c) and 2-week-old (d) P28.11 plants under non-stress conditions (Normal) and after 2 weeks of recovery from heat stress (Recover). (e-g) Relative water content (e), chlorophyll content (f) and proline content (g) of 2-week-old P28.11 plants before stress treatment (Normal), immediately after stress treatment (Heat), and after 1 week of recovery from heat stress (Recover). Values represent means ± standard deviation (n=3). Different letters indicate significant differences between P28.11 and wild-type (analysis of variance, P<0.05). [Click here to view] |
These enhanced physiological responses to heat stress were accompanied by significant changes in proline metabolism. Notably, P28.11 exhibited significantly higher proline accumulation than WT plants both before and after stress treatment [Figure 3g]. These findings suggest that the OsProDH deletion mutation may have resulted in a loss of OsProDH function, leading to enhanced proline accumulation in BT7 rice plants. This increased proline content likely contributes to improved water retention and chlorophyll protection, thereby maintaining photosynthetic apparatus integrity and enhancing the survival of BT7 rice plants under heat-stress conditions.
3.3. OsProDH Mutation Involves in Decreased ROS Damage in Heat Stress
Abiotic stresses, including heat stress, are often associated with oxidative stress [24]. We evaluated the levels of MDA, superoxide radicals, and antioxidant enzyme activities (CAT, POD, and APX) in the OsProDH mutant BT7 rice line P28.11 and WT plants. Heat stress-induced greater ROS damage in WT compared to P28.11, as evidenced by higher levels of superoxide radicals [Figure 4a and b] and MDA [Figure 4c] after stress treatment. No significant differences in these parameters were observed between WT and P28.11 before stress treatment. Interestingly, enzymatic analysis revealed differential changes in antioxidant enzyme activities when rice plants were exposed to heat stress. CAT activity was more strongly induced in P28.11 than in WT under heat stress [Figure 4d]. In contrast, POD [Figure 4e] and APX [Figure 4f] activities showed no significant differences before and after heat stress exposure in both rice lines. These results suggest that the reduced ROS damage in the edited BT7 line may be primarily attributed to increased CAT activity, potentially resulting from enhanced protection conferred by higher proline accumulation compared to WT plants.
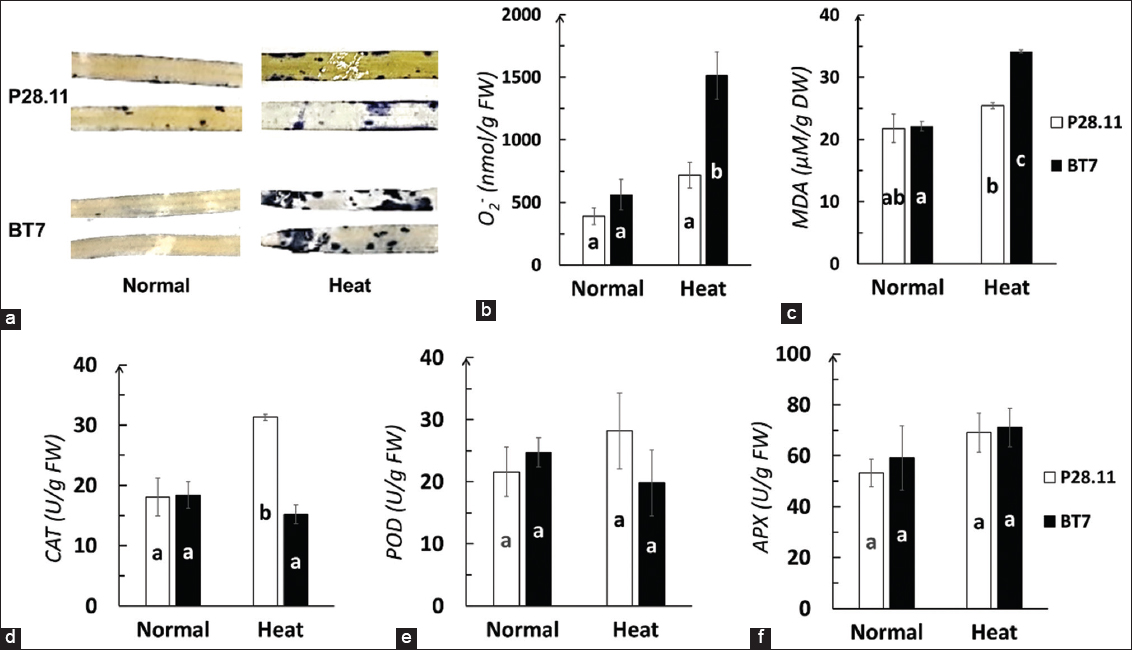 | Figure 4: The heat-induced oxidative stress in Wild-type (WT) and edited BT7 seedlings. Measurements in leaves before (Normal) and after (Heat) stress treatment: (a) NBT staining of leaves (blue spots indicate O2- accumulation); (b) O2- contents; (c) malondialdehyde content; (d-f) activities of catalase, peroxidase and ascorbate peroxidase. Values represent means ± standard deviation (n=3). Different letters indicate significant differences between P28.11 and WT (analysis of variance, P<0.05). [Click here to view] |
3.4. OsProDH Mutation Does Not Affect the Main Agronomic Traits of BT7 Edited Plants
To assess the potential impact of the OsProDH mutation on other agronomic traits of BT7, we evaluated the growth and reproduction of the homozygous mutant T2 line P28.11 under greenhouse conditions. Statistical analysis of variance revealed no significant differences between the gene-edited rice line and the WT line in terms of the growth period, plant height, number of tillers per plant, number of filled grains per panicle, individual yield, and amylose content [Table 2]. These results demonstrate that the 39-nucleotide deletion mutation induced by CRISPR/Cas9 in OsProDH does not significantly affect the important agronomic traits of BT7 under normal growing conditions, suggesting that the enhanced heat tolerance was achieved without compromising other desirable characteristics of the cultivar.
Table 2: Agronomic traits evaluation of homozygous T2 mutant line P28.11.
| Lines | Time duration (day) | Plant height (cm) | No. of tiller per plant | No. of filled grains per panicle | Individual yield (gr) | Amylose content (%) |
|---|---|---|---|---|---|---|
| WT | 102.8±2.17a | 100.4±2.3a | 6.2±0.84a | 72.2±5.26a | 17.06±0.72a | 13.74±0.15a |
| P28.11 | 103.4±3.21a | 100±1.87a | 6±0.71a | 72.2±4.76a | 16.9±0.7a | 13.67±0.13a |
Five plants per line were measured. Data represent the mean values from three independent experiments, with SD shown. Means followed by the same letter do not differ significantly (P<0.05). SD: Standard deviation, WT: Wild-type.
4. DISCUSSION
CRISPR/Cas9 gene editing technology has been widely applied to various crops, with a significant focus on model plants. In recent years, several commercial rice varieties have been successfully improved for biotic stress tolerance and pathogen resistance [14,17,25-28]. CRISPR-Cas9-mediated OsDST gene editing has also improved drought and salt tolerance in the indica mega rice cultivar MTU1010 [29]. However, studies focusing on improving temperature tolerance, particularly in commercial rice varieties have been notably scarce [12]. This gap in research is significant, given the increasing threat of climate change to global rice production. Our study addresses this critical need by targeting the OsProDH gene in the BT7 rice variety, a high-quality cultivar popular in Vietnam but susceptible to bacterial leaf blight and adverse environmental factors such as drought and heat stress. Using CRISPR/Cas9, we developed a homozygous OsProDH mutant BT7 line with improved heat tolerance at seedling and tillering stages while maintaining key agronomic traits comparable to the WT. The success in improving the heat tolerance of a commercial rice variety without compromising its agronomic performance underscores the immense potential of CRISPR/Cas9 technology for climate change-resilient rice breeding programs. This approach offers a precise and efficient method for enhancing specific traits in elite varieties, potentially accelerating the development of climate-adaptive rice cultivars. Our findings have significant implications for rice breeding programs in Vietnam and other rice-producing countries facing similar climate challenges.
Increasing proline accumulation, a well-known osmoprotectant involved in various plant stress responses, is a widely adopted strategy to improve crop stress tolerance [30,31]. Proline content under stress is regulated by balancing synthesis, controlled by P5CS and P5CR enzymes, and degradation, mediated by ProDH [11]. While many studies have successfully enhanced crop stress tolerance by overexpressing P5CS [30,31] or P5CR [32], the resulting genetically modified organisms (GMOs) face regulatory challenges in many countries. Consequently, increasing proline accumulation through ProDH inactivation using gene editing technology presents a potentially more acceptable approach for many countries, including Vietnam [30]. However, research on improving crop stress tolerance by knocking out or inhibiting ProDH expression remains limited [12]. This scarcity may be attributed to the heterogeneous role of ProDH in stress responses, particularly under heat stress. For instance, OsProDH expression decreases under heat stress in japonica rice varieties Nipponbare and Kongyu13 [11,12], but increases in the indica rice variety BRRI-53 [11]. In our study, the deletion of 13 amino acids in the functional domain of OsProDH enhanced proline accumulation in BT7 rice plants both before and after heat stress treatment, subsequently improving their heat stress tolerance. These findings align with the results of OsProDH gene editing in the japonica rice variety Kongyu13 [12]. In that study, Guo et al. (2020) demonstrated that OsProDH, located in mitochondria, controls H2O2 levels through proline metabolism and negatively regulates heat tolerance in rice. This consistency across different rice varieties suggests that the role of OsProDH in heat tolerance is likely conserved across rice subspecies despite their genetic differences. Our results, combined with previous evidence, demonstrate that editing the ProDH gene is an effective strategy for developing new rice varieties with enhanced heat tolerance.
Having demonstrated the effectiveness of OsProDH editing in enhancing heat tolerance in BT7 rice, it is important to consider how this targeted gene editing approach compares with conventional breeding strategies for crop improvement. The development of heat-tolerant rice varieties through CRISPR/Cas9-mediated gene editing of the OsProDH gene in the Bacthom 7 cultivar demonstrates significant advantages over traditional breeding and transgenic methods. While conventional approaches have successfully developed heat-tolerant varieties, they often involve lengthy processes and may introduce undesirable traits. Our CRISPR/Cas9 approach allows for precise modification, resulting in improved proline accumulation and enhanced heat tolerance without affecting other agronomic traits. The efficacy of CRISPR/Cas9 in precisely modifying target genes for enhanced stress tolerance has been demonstrated in multiple studies. Guo et al. (2020) showed that targeted modifications of OsProDH could alter proline metabolism pathways specifically without disrupting other cellular processes. This specificity is crucial for complex traits like heat tolerance, which involves multiple physiological pathways. Traditional methods struggle to isolate and enhance specific heat tolerance mechanisms without impacting other plant processes. Our gene editing approach enables targeted enhancement while maintaining the cultivar’s genetic integrity. CRISPR/Cas9 significantly reduces development time to 3–5 years, compared to 10–15 years for traditional breeding, which is critical in the face of accelerating climate change. Decreased ROS damage in heat-stressed edited plants highlights the ability to fine-tune specific physiological responses. In addition, from a regulatory perspective, CRISPR-edited crops often face less stringent GMO management policies than transgenic varieties. Many countries classify gene-edited crops without foreign DNA as non-GMO, potentially streamlining their approval and commercialization. This regulatory advantage could accelerate the adoption of heat-tolerant rice varieties developed through CRISPR/Cas9 editing, addressing food security concerns more rapidly. While conventional breeding and transgenic methods remain relevant, our study underscores the power, precision, and regulatory advantages of gene editing technologies in rapidly developing rice varieties with enhanced heat tolerance, which is crucial for ensuring food security in a warming world. Future research should focus on field trials, multi-stress tolerance, and comprehensive ecological assessments to fully realize the potential of this gene editing strategy in rice improvement programs worldwide.
5. CONCLUSION
In this study, we successfully employed CRISPR/Cas9 gene editing technology to modify the OsProDH gene in the BT7 rice variety, resulting in enhanced heat stress tolerance. The 39-nucleotide deletion in OsProDH led to increased proline accumulation, improved water retention, and better photosynthetic protection under heat-stress conditions. Notably, the edited plants exhibited reduced ROS damage and increased CAT activity, contributing to their superior heat tolerance.
Our findings demonstrate that targeted modification of OsProDH is an effective strategy for developing heat-tolerant rice varieties without compromising essential agronomic traits. This approach offers several advantages over traditional breeding methods, including precision, efficiency, and the potential for rapid variety development. The success in improving BT7’s heat tolerance while maintaining its desirable characteristics underscores the potential of gene editing technologies in addressing the challenges posed by climate change to rice production.
Future research should focus on field trials to validate the performance of these heat-tolerant lines under various environmental conditions and to assess any potential ecological impacts. Additionally, exploring the combination of OsProDH editing with other stress-tolerance traits could further enhance the resilience of rice varieties to multiple abiotic stresses. This study lays the groundwork for developing climate-resilient rice varieties, contributing to global food security efforts in the face of rising temperatures and changing climate patterns.
6. ACKNOWLEDGMENTS
This research was funded by the Vietnam Ministry of Science and Technology (project code ?T?L.CN-52/22). We extend our gratitude to Msc. Nguyen Thi Thu Ha (Institute of Agricultural Genetics, Vietnam) for rice transgenic screening, Dr. Nguyen Tien Dung and his team (Thai Nguyen University of Agriculture and Forestry, Vietnam) for conducting agronomic experiments, Dr. Sebastien Cunnac (Research Institute for Development, France) for providing vectors, and Thaibinh Seed Corporation for supplying BT7 seeds.
7. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. CONFLICT OF INTEREST STATEMENT
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
11. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
12. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Roy SC, Shil P. Assessment of genetic heritability in rice breeding lines based on morphological traits and caryopsis ultrastructure. Sci Rep 2020;10:7830. [CrossRef]
2. Huang F, Lei Y, Duan J, Kang Y, Luo Y, Ding D, et al. Investigation of heat stress responses and adaptation mechanisms by integrative metabolome and transcriptome analysis in tea plants (Camellia sinensis). Sci Rep 2024;14:10023. [CrossRef]
3. Xing YH, Lu H, Zhu X, Deng Y, Xie Y, Luo Q, et al. How rice responds to temperature changes and defeats heat stress. Rice (N Y) 2024;17:73. [CrossRef]
4. Li S, Fleisher DH, Barnaby JY. Quantifying the impact of climate change and extreme heat on rice in the United States. Agric For Meteorol 2024;355:110145. [CrossRef]
5. Zhao C, Liu B, Piao S, Wang X, Lobell DB, Huang Y, et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc Natl Acad Sci U S A 2017;114:9326-31. [CrossRef]
6. Yang Y, Yu J, Qian Q, Shang L. Enhancement of heat and drought stress tolerance in rice by genetic manipulation:A systematic review. Rice (N Y) 2022;15:67. [CrossRef]
7. Sam VH, Van PT, Ha NT, Ha NT, Huong PT, Hoi PX, et al. Design and transformation of OsSWEET14-editing T-DNA construct into bacthom 7 rice cultivar. Vietnam Acad J Biol 2021;43:99-108. [CrossRef]
8. Le TN, Nguyen TM, Nguyen BN, Nguyen TN, Pham TT, Le HL, et al. Improvement of resistance to bacteria blight, blast diseases and brown plant hopper of Bac Thom 7 rice variety by molecular markers. J Vietnam Agric Sci Technol 2025;1:96-104.
9. Hosseinifard M, Stefaniak S, Ghorbani Javid M, Soltani E, Wojtyla ?, Garnczarska M. Contribution of exogenous proline to abiotic stresses tolerance in plants:A review. Int J Mol Sci 2022;23:5186. [CrossRef]
10. Meena M, Divyanshu K, Kumar S, Swapnil P, Zehra A, Shukla V, et al. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019;5:e02952. [CrossRef]
11. Arabia S, Shah MN, Sami AA, Ghosh A, Islam T. Identification and expression profiling of proline metabolizing genes in Arabidopsis thaliana and Oryza sativa to reveal their stress-specific transcript alteration. Physiol Mol Biol Plants 2021;27:1469-85. [CrossRef]
12. Guo M, Zhang X, Liu J, Hou L, Liu H, Zhao X. OsProDH negatively regulates thermotolerance in rice by modulating proline metabolism and reactive oxygen species scavenging. Rice (N Y) 2020;13:61. [CrossRef]
13. Liu H, Ding Y, Zhou Y, Jin W, Xie K, Chen LL. CRISPR-P 2.0:An improved CRISPR-Cas9 tool for genome editing in plants. Mol Plant 2017;10:530-2. [CrossRef]
14. Oliva R, Ji C, Atienza-Grande G, Huguet-Tapia JC, Perez-Quintero A, Li T, et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat Biotechnol 2019;37:1344-50. [CrossRef]
15. Cecchini NM, Monteoliva MI, Alvarez ME. Proline dehydrogenase contributes to pathogen defense in Arabidopsis. Plant Physiol 2011;155:1947-59. [CrossRef]
16. Monteoliva MI, Rizzi YS, Cecchini NM, Hajirezaei MR, Alvarez ME. Context of action of proline dehydrogenase (ProDH) in the hypersensitive response of arabidopsis. BMC Plant Biol 2014;14:21. [CrossRef]
17. Chen J, Miao Z, Kong D, Zhang A, Wang F, Liu G, et al. Application of CRISPR/Cas9 technology in rice germplasm innovation and genetic improvement. Genes (Basel) 2024;15:1492. [CrossRef]
18. Stemmer M, Thumberger T, Del Sol Keyer M, Wittbrodt J, Mateo JL. CCTop:An intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS One 2015;12:e0176619. [CrossRef]
19. Dai TL, Huong PT, Quyen CL, Cuu NV, Ha NT, Hoi PX, et al. Design of T-DNA construct for editing SWEET genes involved bacterial leaf blight disease in TBR225 rice variety. Vietnam J Agric Rural Dev 2022;11:11-8.
20. Ma X, Chen L, Zhu Q, Chen Y, Liu YG. Rapid decoding of sequence-specific nuclease-induced heterozygous and biallelic mutations by direct sequencing of PCR products. Mol Plant 2015;8:1285-7. [CrossRef]
21. Vijayaraghavareddy P, Vemanna RS, Yin X, Struik PC, Makarla U, Sreeman S. Acquired traits contribute more to drought tolerance in wheat than in rice. Plant Phenomics 2020;2020:5905371. [CrossRef]
22. Yadav A, Singh A, Rastogi A, Gaur VS, Yadav MK, Birla H, et al. Plasticity of OsERF109 mitigates drought stress by modulating the antioxidant defense system and morphophysiological traits in rice. Sci Rep 2023;13:10445.
23. Li R, Liu C, Zhao R, Wang L, Chen L, Yu W, et al. CRISPR/Cas9-mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Biol 2019;19:38. [CrossRef]
24. You J, Chan Z. ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 2015;6:1092. [CrossRef]
25. Duy PN, Lan DT, Pham Thu H, Thi Thu HP, Nguyen Thanh H, Pham NP, et al. Improved bacterial leaf blight disease resistance in the major elite Vietnamese rice cultivar TBR225 via editing of the OsSWEET14 promoter. PLoS One 2021;16:e0255470. [CrossRef]
26. Li C, Zhou L, Wu B, Li S, Zha W, Li W, et al. Improvement of bacterial blight resistance in two conventionally cultivated rice varieties by editing the noncoding region. Cells 2022;11:2535. [CrossRef]
27. Wang F, Wang C, Liu P, Lei C, Hao W, Gao Y, et al. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS One 2016;11:e0154027. [CrossRef]
28. Zhou Y, Xu S, Jiang N, Zhao X, Bai Z, Liu J, et al. Engineering of rice varieties with enhanced resistances to both blast and bacterial blight diseases via CRISPR/Cas9. Plant Biotechnol J 2022;20:876-85. [CrossRef]
29. Santosh Kumar VV, Verma RK, Yadav SK, Yadav P, Watts A, Rao MV, et al. CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol Mol Biol Plants 2020;26:1099-110. [CrossRef]
30. Kaur G, Asthir B. Proline:A key player in plant abiotic stress tolerance. Biol Plant 2015;59:609-19. [CrossRef]
31. Zhu B, Su J, Chang M, Verma DP, Fan Y, Wu R. Overexpression of a Δ1-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water- and salt-stress in transgenic rice. Plant Sci 1998;139:41-8. [CrossRef]
32. Chen C, Cui X, Zhang P, Wang Z, Zhang J. Expression of the pyrroline-5-carboxylate reductase (P5CR) gene from the wild grapevine Vitis yeshanensis promotes drought resistance in transgenic Arabidopsis. Plant Physiol Biochem 2021;168:188-201. [CrossRef]