1. INTRODUCTION
Antimicrobial resistance (AMR) is a growing global health threat that occurs when microorganisms, including bacteria, fungi, and parasites, develop the ability to resist the effects of antimicrobial drugs that were previously effective against them. This resistance emerges primarily due to genetic mutations and the transfer of resistance genes through horizontal or vertical gene transfer [1,2]. The misuse and overuse of antibiotics in healthcare, agriculture, and animal husbandry have accelerated this process [3,4]. As a result, multidrug-resistant and extensively drug-resistant infections have become more common, complicating treatment and leading to prolonged hospital stays, increased mortality, and rising healthcare costs [5,6]. Bacteria can acquire resistance through genetic mutations that modify drug targets or by obtaining resistance genes from other resistant species via horizontal gene transfer (HGT). These mechanisms enable pathogens to survive antimicrobial treatments, making infections harder to control and increasing the risk of severe complications and death in affected individuals [7,8]. The increasing prevalence of AMR highlights the urgent need for early and precise detection. Identifying resistance markers in pathogens at an early stage enables healthcare providers to prescribe the most effective antibiotics, reducing the misuse of broad-spectrum drugs and slowing the development of further resistance. However, conventional AMR detection methods, including culture-based techniques, molecular assays, and phenotypic tests, have significant limitations [9]. Culture-based methods involve isolating pathogens from clinical samples and exposing them to various antimicrobial agents to assess growth inhibition. Common techniques used in clinical microbiology laboratories include disk diffusion and broth microdilution assays [10]. However, these methods require 48–72 h to yield results, delaying treatment decisions and increasing the risk of complications. In addition, they demand specialized laboratory equipment and trained personnel, which may not be readily available in resource-limited settings [11].
Molecular approaches, such as polymerase chain reaction (PCR)-based diagnostics, provide faster results by targeting specific genetic markers linked to AMR [12]. For example, PCR can detect resistance genes such as the mecA gene in Staphylococcus aureus, which confers methicillin resistance, or the blaNDM gene in Enterobacteriaceae (e.g., Klebsiella pneumoniae and Escherichia coli), which encodes carbapenemase and leads to resistance against carbapenem antibiotics [13]. While PCR procedures can produce faster results than culture-based techniques, they still require specialized equipment, such as thermal cyclers, and are often more expensive and labor intensive [14]. Despite advancements, traditional diagnostic methods remain limited by factors such as laboratory infrastructure requirements, long turnaround times, and high costs [15]. To address these challenges, novel diagnostic technologies have emerged, offering faster, more accurate, and cost-effective AMR detection. These include loop-mediated isothermal amplification (LAMP), nanopore sequencing, and biosensor-based approaches, which enhance detection speed and portability. Among these, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-based diagnostics have gained significant attention due to their high specificity, rapid turnaround time, and potential for point-of-care applications.
2. CRISPR TECHNOLOGY: AN OVERVIEW
CRISPR is a naturally occurring immunological system found in bacteria and archaea that allows these microorganisms to recognize and defend against invading viruses, including bacteriophages. This system is made up of small, repetitive DNA sequences separated by viral DNA pieces known as “spacers.” These spacers act as a genetic memory of earlier viral infections, allowing bacteria and archaea to recognize and resist against future attacks by the viruses. The CRISPR system consists of two key components: DNA sequences (CRISPR arrays) and CRISPR-associated proteins (Cas proteins), such as Cas9, Cas12, and Cas13, which play an important role in the immune response [16,17]. The CRISPR-Cas system can recognize and cut specific DNA or RNA sequences. Cas9, the most well-known CRISPR-associated protein, acts as a molecular “scissors” by cutting double-stranded DNA at specified target places [18]. The Cas9 protein is steered to its target by a short RNA molecule known as the guide RNA (gRNA), which is designed to be complementary to a specific DNA sequence. This allows the CRISPR system to be configured for precise genomic editing, which has revolutionized genetic engineering and biotechnology. Newer Cas proteins, such as Cas12 and Cas13, have broadened the possible applications of CRISPR technology. Cas12 targets DNA, while Cas13 targets RNA, providing greater versatility in diagnostics and disease detection [19,20].
In recent years, the CRISPR-Cas system has been used for a range of applications other than gene editing, such as diagnostics and disease detection. One of the most intriguing applications of CRISPR is its capacity to detect certain genomic sequences, such as those linked to AMR indicators in pathogens. CRISPR-based diagnostic tools have been created to discover AMR-related genes, which are frequently responsible for bacteria becoming resistant to conventional antibiotics. These diagnostic methods take advantage of the extremely specific recognition of DNA or RNA sequences that correlate to AMR indicators, enabling for the quick detection of resistant bacteria [21]. The CRISPR-Cas system identifies and binds to a specific genetic sequence within a pathogen’s genome. When the Cas protein binds, it undergoes a conformational change, activating its enzymatic activity. This activation can initiate downstream detecting systems, resulting in a detectable signal. For example, the Cas12 and Cas13 proteins have collateral cleavage activity, which means that once bound to their target, they can break neighboring reporter molecules, resulting in signals such as fluorescence, luminescence, or color changes. These signals are easily detectable and quantifiable, allowing the identification of AMR indicators in clinical samples [22,23].
One of the key advantages of using CRISPR for diagnosis is the ability to detect low amounts of target DNA or RNA with high sensitivity and specificity. CRISPR-based diagnostic platforms can detect AMR markers even in cases where the pathogen is present in low quantities or when there is a mixed infection, offering a more reliable and accurate method than traditional culture-based or PCR-based tests [24]. In addition, CRISPR diagnostics can be tailored to identify a wide range of AMR-related genes, allowing for multiplexed testing, where multiple resistance markers can be detected simultaneously in a single assay. This flexibility makes CRISPR a highly adaptable and powerful tool for pathogen detection and AMR surveillance [25]. The CRISPR technology’s key aspects and its application in pathogen detection, particularly for AMR [Table 1].
Table 1: Overview of CRISPR technology and its applications in pathogen detection and antimicrobial resistance.
| Aspect | Description | References |
|---|---|---|
| Pathogen detection | CRISPR-based diagnostics (e.g., SHERLOCK, DETECTR) enable rapid and precise detection of bacterial and viral infections, including antimicrobial resistance (AMR) markers. | [16] |
| Antimicrobial resistance (AMR) detection | CRISPR-Cas systems identify AMR genes in pathogens, allowing early detection of drug-resistant infections and guiding effective treatment decisions. | [26] |
| Gene editing | CRISPR-Cas9 allows precise modification of DNA sequences, enabling corrections of genetic mutations responsible for diseases such as sickle cell anemia and cystic fibrosis. | [27] |
| Therapeutic applications | CRISPR is being explored for treating genetic disorders, cancers, and infectious diseases by targeting and modifying disease-causing genes. | [28] |
| Viral disease treatment | CRISPR has shown promise in targeting and disrupting viral genomes, such as HIV and hepatitis B, potentially leading to novel antiviral therapies. | [29] |
| Agricultural and industrial use | CRISPR enhances crop resistance to pests, improves yield, and aids in developing probiotic bacterial strains with beneficial properties. | [30] |
3. CRISPR-BASED DIAGNOSTIC FOR (AMR) DETECTION
Several CRISPR-based diagnostic platforms have been created to detect AMR markers in pathogens, significantly improving the speed and accuracy of pathogen detection. These platforms take advantage of the CRISPR-Cas system’s accuracy and programmability, which may be tailored to target specific AMR-related genomic regions. Cas9, one of the most widely utilized CRISPR proteins for diagnostics, chops DNA at a specific target location. CRISPR-Cas9 is used in AMR gene detection beyond its role as a molecular scissor by acting as a highly specific sequence recognition tool. Instead of cleaving DNA, a catalytically inactive Cas9 (dCas9) binds to AMR genes without cutting, allowing precise identification of resistance markers. This binding can be coupled with fluorescence or colorimetric assays to produce visual indicators of AMR presence. In biosensor applications, dCas9 can be linked to fluorescent reporters, enabling real-time visualization of AMR genes under a fluorescence microscope. Colorimetric detection methods use dCas9 to trigger a visible color change when bound to a target AMR gene, facilitating easy identification. Electrochemical detection integrates dCas9 with electrodes that generate an electrical signal on AMR sequence recognition, providing rapid and sensitive detection in clinical settings. CRISPR-Cas9 is also integrated into diagnostic platforms like Specific High-sensitivity Enzymatic Reporter UnLOCKing (SHERLOCK), originally designed for RNA detection with Cas13 but adaptable for DNA-based AMR detection. These platforms utilize Cas9’s sequence-targeting ability to activate enzymatic reporters, amplifying detection signals for highly sensitive pathogen identification. By leveraging these approaches, CRISPR-Cas9 enables rapid, accurate, and scalable AMR gene detection without necessarily altering the genetic material [31].
An improved form, the CRISPR-Cas12 system, provides additional advantages, such as increased collateral cleavage activity. This means that once Cas12 binds to its target DNA, it can cut neighboring single-stranded DNA (ssDNA) at random, activating a reporter molecule and producing a visible signal such as fluorescence or colorimetric changes. Fluorescence in CRISPR-Cas12-based AMR detection comes from reporter molecules that are activated upon collateral cleavage. When Cas12 binds to its target AMR gene, it undergoes non-specific ssDNA cleavage, cutting nearby fluorescently labeled probes. A common approach uses a fluorescent-quencher probe, which consists of a fluorophore and a quencher linked by an ssDNA strand. In its intact state, the quencher suppresses fluorescence. When Cas12 cleaves the probe, the fluorophore separates from the quencher, producing a detectable fluorescence signal. CRISPR-Cas12’s high sensitivity and rapid reaction make it a perfect candidate for point-of-care testing, which requires speedy findings [32]. Another intriguing CRISPR tool, Cas13, targets RNA rather than DNA, potentially broadening the scope of CRISPR diagnostics to encompass RNA viruses and other infections with RNA-based genomes [33]. Cas13-based diagnostics are especially useful for detecting AMR in viral infections, allowing for greater flexibility in pathogen surveillance. Incorporating CRISPR technology with lateral flow assays (LFA) improves diagnostic capabilities by allowing for simple, user-friendly tests with little equipment. These assays, which are frequently used for point-of-care testing, enable healthcare practitioners to quickly screen for AMR indicators in clinical samples, allowing for more timely treatment decisions [34]. Furthermore, CRISPR-based diagnostics can be integrated with isothermal amplification methods such as Recombinase Polymerase Amplification and LAMP. These approaches enable the amplification of target DNA or RNA without the need for thermal cycling, making the platform ideal for resource-constrained environments and enabling quick, on-site testing [35].
Another notable feature of CRISPR-based diagnostic tools is their capacity to detect many AMR indicators concurrently via multiplexing. This capacity not only improves testing efficiency but also broadens the detection scope, enabling the identification of numerous resistance mechanisms in a single sample. This is especially significant in cases of polymicrobial illnesses, where many resistant organisms may exist [36]. Overall, CRISPR-based diagnostic tools provide a highly sensitive, quick, and versatile option for AMR detection, with the potential to transform clinical diagnosis and enhance the management of resistant microbes globally. Various CRISPR-based diagnostic platforms utilize different mechanisms to detect AMR markers in pathogens, including collateral cleavage activity, fluorescence-based detection, and colorimetric assays. These methods enhance sensitivity and enable rapid identification of resistance genes in clinical samples [Figure 1].
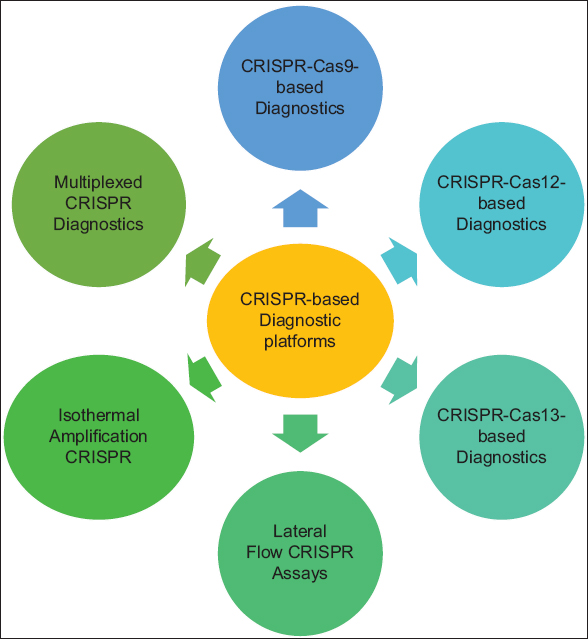 | Figure 1: Clustered regularly interspaced short palindromic repeats-based diagnostic platforms for antimicrobial resistance detection. [Click here to view] |
3.1. SHERLOCK
SHERLOCK is a revolutionary CRISPR-based diagnostic tool that uses Cas13, an RNA-targeting CRISPR protein, to detect nucleic acids, specifically RNA. When led by a specific RNA sequence, Cas13 initiates its unique collateral RNA cleavage function. This means that when Cas13 binds to its target RNA, it not only cleaves it but also indiscriminately cuts adjacent RNA molecules, resulting in a visible signal. This signal can be visual, such as a color change, or quantifiable, such as fluorescence. However, detecting fluorescence signals requires specialized technology, such as a computer-based system or a fluorescence reader, to accurately measure and interpret the emitted signals when the adjacent gene is cleaved [37,38]. SHERLOCK has showed promising results in the detection of AMR indicators. CRISPR-based diagnostics identify antibiotic resistance markers by designing CRISPR RNA (crRNA) sequences that specifically recognize RNA transcripts of resistance genes. When the CRISPR-Cas system detects the target RNA, it activates a signal, such as fluorescence or a color change, indicating the presence of the resistance gene. This allows for rapid and precise identification of antibiotic-resistant pathogens in clinical samples. This tool is especially useful for finding resistance mechanisms in real time, allowing healthcare providers to make faster treatment decisions [39]. The SHERLOCK platform is very sensitive, detecting even low amounts of target RNA, making it especially helpful in clinical and field settings where rapid diagnostics are required [40].
One of the primary benefits of SHERLOCK is its mobility and ease of use. The device can be configured for point-of-care testing, enabling on-site AMR detection in settings lacking modern laboratory facilities. This is especially useful in low-resource settings, where early diagnosis of resistant microorganisms can minimize unnecessary antibiotic use and limit the spread of resistant microbes [41]. In addition, SHERLOCK’s adaptability goes beyond bacterial pathogens. It has also been tested for the detection of additional infectious diseases, which increases its potential for wider application in global health monitoring and AMR surveillance [42].
3.2. DNA Endonuclease Targeted CRISPR Trans Reporter (DETECTR)
DETECTR is a CRISPR-based diagnostic platform that relies on the Cas12 protein, a CRISPR-associated protein renowned for its capacity to detect and bind to specific DNA sequences. Cas12’s collateral cleavage is activated only after binding to a specific AMR gene sequence, ensuring high specificity. The gRNA (crRNA) is designed to target unique regions, minimizing off-target effects. Collateral cleavage primarily affects ssDNA reporters, not genomic DNA, and requires a PAM sequence for activation. In addition, Cas12 is mostly used in vitro for diagnostics, preventing unintended damage to the host genome. The reporter can generate a detectable signal, such as fluorescence or a colorimetric change, which gives a quick and obvious indicator of the presence of the target DNA sequence [43,44]. DETECTR is frequently used for the detection of infections and AMR indicators. The technique is especially successful at identifying resistance genes, including those responsible for beta-lactam antibiotic resistance, such as penicillin, as well as resistance to other antibiotic classes like aminoglycosides. DETECTR, which targets specific DNA sequences linked with these resistance genes, can swiftly identify resistant bacteria strains, allowing healthcare providers to make more accurate treatment decisions. The technology is highly sensitive, detecting even trace amounts of target DNA, and specific enough to distinguish between diseases with various resistance profiles [31,45]. One of the primary benefits of DETECTR is its quick response time. Results can be acquired in as little as 30 min, making it suitable for point-of-care testing when timely results are critical for optimal patient management. This is especially significant in clinical settings because early detection of resistant organisms can limit the wasteful use of broad-spectrum antibiotics, hence preventing further resistance development. A major drawback of applying and extending CRISPR-based technology in resource-scarce areas is the need for well-trained personnel to handle sample preparation, assay execution, and result interpretation. In addition, despite being more affordable than traditional methods, the technology still requires financial resources for reagents, portable detection devices, and infrastructure to maintain cold chain storage for CRISPR components, which can be challenging in low-resource settings [46].
3.3. CRISPR-based LFAs
CRISPR-based LFAs are a big step forward in point-of-care diagnostics, combining the simplicity and quick findings of standard lateral flow technologies with the precision and programmability of CRISPR-Cas. LFAs are widely utilized in a variety of diagnostic applications, including pregnancy tests and rapid antigen detection, because of their simplicity, mobility, and quick turnaround times [47]. The addition of CRISPR technology to existing assays has increased their capability, allowing for the rapid and reliable identification of AMR indicators directly from clinical samples such as urine, sputum, or blood [48]. Cas12 and Cas13 are CRISPR-associated proteins used in diagnostics to detect AMR genes. Cas12 targets DNA and, upon binding, activates collateral cleavage, cutting nearby ssDNA reporters to generate a fluorescence or colorimetric signal. Cas13 targets RNA and exhibits similar collateral cleavage, making it ideal for detecting RNA-based resistance markers. These properties enable CRISPR-based LFAs for rapid and portable AMR detection. When these proteins detect their target sequences, they trigger a distinct collateral cleavage activity that cleaves neighboring reporter molecules. These reporter molecules are frequently attached to colorimetric or fluorescent tags, which emit a detectable signal upon cleavage, usually apparent as a color shift on the test strip [49,50].
A CRISPR-based LFA involves introducing clinical samples to a test strip containing an area where the CRISPR reaction occurs. If the sample contains AMR genes, the CRISPR system will bind to the target sequences and cause the cleavage of reporter molecules, resulting in a visible color shift or signal on the test strip. This method enables the quick detection of AMR markers without the requirement for complex laboratory processing, making it a suitable tool for use in resource-constrained settings or on-site testing in clinical contexts. The assay’s simplicity and quickness make it widely accessible, allowing healthcare providers to make more informed treatment decisions in real time [47]. One of the primary benefits of CRISPR-based LFAs is their capacity to detect a wide variety of AMR indicators with excellent sensitivity and specificity. The CRISPR system’s precision in targeting genetic sequences ensures that only pathogens with specific resistance genes are discovered, reducing the chance of false positives. Furthermore, CRISPR-based LFAs can be multiplexed to detect numerous AMR markers at the same time, making diagnostic testing more efficient. This is especially important when illnesses are caused by many pathogens or when different types of resistance genes must be found simultaneously [51].
The use of CRISPR-based LFAs in clinical settings provides significant advantages over standard AMR detection techniques. Conventional approaches, such as culture-based techniques or PCR, can take hours or days to produce findings and frequently necessitate specialized laboratory equipment and skilled workers. In contrast, CRISPR-based LFAs provide results significantly faster, frequently in 30 min or less. This speed is critical in cases when a prompt diagnosis is required to guide treatment decisions and prevent the spread of resistant diseases [52,53]. Furthermore, the portability of CRISPR-based LFAs makes them ideal for use in field settings where access to advanced laboratory infrastructure is limited. These assays can be employed in remote or low-resource settings, making them an affordable option for monitoring AMR in a variety of populations. They also have the potential to be used in monitoring programs to track the transmission of resistance genes across different environments.
3.4. CRISPR-Cas9 for Genomic Editing and AMR Surveillance
CRISPR-Cas9, a groundbreaking genetic engineering technique, has expanded its applications beyond gene editing to include genomic surveillance of AMR. The CRISPR-Cas9 system, which was originally discovered as a bacterial immunity mechanism, allows for precise and targeted alterations to DNA sequences in living species, including bacteria. This capacity to directly modify the bacterial genome has been extremely useful for investigating and identifying specific resistance genes in clinical isolates. CRISPR-Cas9 allows for a more detailed analysis of how resistance characteristics are acquired and spread among bacterial populations, which is critical for understanding and controlling the global AMR epidemic [54,55]. One important application of CRISPR-Cas9 in AMR surveillance is to track the evolution of novel resistance mechanisms in real time. When researchers use CRISPR-based genome editing on a bacterial isolate with a known resistance profile, they can target and deactivate specific AMR genes, such as those that give resistance to antibiotics like beta-lactams or aminoglycosides. Observing changes in the bacterial phenotype following such alterations allows researchers to validate the significance of certain genes in resistance and track how these genes evolve or spread across other bacterial strains [56,57]. This sort of surveillance aids in predicting the dynamics of AMR in varied situations and can lead to more targeted measures to restrict the spread of resistance. In addition, CRISPR-Cas9 has been used with next-generation sequencing (NGS) to improve AMR surveillance. NGS enables thorough, high-throughput sequencing of whole bacterial genomes, offering a detailed understanding of genetic alterations and resistance patterns found in complex bacterial populations [58]. When combined with CRISPR-Cas9, NGS may precisely identify AMR-associated genomic alterations, even in mixed bacterial populations. This combination enables researchers to not only detect resistance genes but also comprehend their genomic context, such as how they cluster with other resistance determinants or evolve throughout distinct bacterial lineages [59]. Such deep insights into bacterial genomes are critical for monitoring resistance patterns, particularly in situations where several diseases coexist and interact. The combination of CRISPR-Cas9 and genomic surveillance tools such as NGS makes it easier to monitor resistance gene transfer between bacteria. HGT is a significant contribution to the transmission of AMR because resistance genes can be passed between bacterial species. By using CRISPR technology to selectively knock out resistance genes in clinical isolates, researchers can study gene transfer dynamics and gain deeper insights into how antibiotic resistance spreads in bacterial populations [60]. Furthermore, CRISPR-Cas9 technology is being investigated for its ability to generate genetic libraries of bacterial strains with specific AMR profiles. Creating these libraries allows researchers to explore the genetic pathways involved in resistance development and identify previously unknown resistance mechanisms. These libraries can also be used to test potential antimicrobial medicines, providing information about how novel drugs may interact with resistant bacteria [57].
4. APPLICATION OF CRISPR DIAGNOSTICS FOR AMR IN DIFFERENT PATHOGENS
CRISPR-based diagnostics for AMR detection rely on the ability of CRISPR-Cas systems to recognize and cleave specific DNA or RNA sequences associated with resistance genes. The process begins with the collection of bacterial samples from clinical sources such as blood, urine, or sputum, followed by the extraction of nucleic acids. A CRISPR-associated enzyme, such as Cas12, Cas13, or Cas9, is programmed with a gRNA to specifically target known AMR genes. Upon successful binding, the enzyme cleaves the target sequence, activating a reporter molecule that generates a detectable signal. This signal can be visualized through fluorescence, colorimetric changes, or lateral flow strips, allowing for rapid and sensitive detection of resistance markers. The speed and accuracy of CRISPR-based diagnostics enable healthcare professionals to quickly identify resistant bacterial strains, facilitating timely and informed decisions regarding antibiotic treatment and reducing the spread of AMR [61]. The application of CRISPR-based diagnostics for AMR in different bacterial pathogens, highlighting the key features, CRISPR systems used, and their clinical relevance [Table 2].
Table 2: Application of CRISPR-based diagnostics for detecting antimicrobial resistance markers in different pathogens.
| Pathogen | Target resistance gene (s) | CRISPR platform used | Detection method | Clinical application | References |
|---|---|---|---|---|---|
| Methicillin-resistant Staphylococcus aureus (MRSA) | Methicillin resistance mecA gene (responsible for beta-lactam resistance) | CRISPR-Cas9 or CRISPR-Cas12 | Fluorescent or colorimetric detection | Rapid MRSA identification for timely antibiotic selection | [56] |
| Carbapenem-resistant Enterobacteriaceae | Carbapenemase-producing genes (e.g., KPC, NDM, VIM, GES, IMP, SPM, SIM, and OXA-48) | CRISPR-Cas12 or CRISPR-Cas13 | Lateral flow assay, fluorescence | Quick detection of carbapenem resistance to guide treatment decisions | [62] |
| Vancomycin-resistant Enterococci (VRE) | vanA and vanB genes (confers resistance to vancomycin) | CRISPR-Cas9 or CRISPR-Cas12 | Colorimetric or fluorescence-based assay | Helps in early detection of VRE to prevent hospital outbreaks | [63] |
| Multidrug-resistant Mycobacterium tuberculosis (MDR-TB) | rpoB gene (rifampin resistance), katG gene (isoniazid resistance) | CRISPR-Cas9 or CRISPR-Cas12 | Fluorescence-based detection, sequencing integration | Accelerates MDR-TB diagnosis and supports proper treatment selection | [64] |
| Escherichia coli and Pseudomonas aeruginosa | Beta-lactamase, aminoglycoside resistance genes | CRISPR-Cas12 or CRISPR-Cas13 | Fluorescence, paper-based biosensors | Enables rapid screening of resistant strains in urinary tract infections and other infections | [65] |
5. ADVANTAGES OF CRISPR-BASED DIAGNOSTICS FOR AMR DETECTION
CRISPR-based diagnostic technologies outperform traditional diagnostic approaches in terms of speed, sensitivity, specificity, and portability, making them valuable tools in both clinical and field settings. The primary benefits are as follows:
5.1. Speed
CRISPR-based diagnostics offer a quick turnaround time, which is a key advantage. Traditional culture-based procedures can take several days to produce results, whereas CRISPR-based diagnostics can yield results in 30 min to an hour. This fast response allows for timely decision-making in clinical settings, allowing appropriate antibiotic administration and improving patient outcomes [28].
5.2. Sensitivity and Specificity
The CRISPR system’s capacity to target specific genomic sequences makes it extremely sensitive and specific for detecting AMR indicators. Even when the pathogen load is minimal, CRISPR-based diagnostics may accurately detect resistance genes, allowing for early diagnosis of infections. This high sensitivity and specificity limit the possibility of false positives or negatives, resulting in reliable detection of resistant infections [66].
5.3. Portability
Many CRISPR-based diagnostic technologies, like LFAs and portable handheld devices, are intended to be lightweight and simple to use in nonlaboratory settings. These platforms can be used at point-of-care venues such as hospitals, clinics, and even field settings, eliminating the need for costly laboratory equipment. This portability is especially useful in resource-constrained environments or during outbreaks, where prompt diagnosis is vital [67].
5.4. Cost-effectiveness
CRISPR-based diagnostics are less expensive than traditional procedures, making them more accessible in resource-limited environments. The CRISPR technique’s simplicity reduces production costs, and many diagnostic platforms do not require expensive chemicals or complicated equipment [46]. This pricing makes wider deployment more feasible, especially in low- and middle-income nations with a high burden of AMR.
6. CHALLENGES AND FUTURE DIRECTIONS
Despite the tremendous promise of CRISPR-based diagnostics for AMR detection, various hurdles must be solved before these technologies can be completely implemented in clinical and field settings.
6.1. Complexity of Sample Preparation
While CRISPR systems can detect AMR indicators directly from clinical samples, the method frequently includes nucleic acid extraction and amplification steps. These stages, while necessary to ensure the quality and quantity of genetic material, can add complexity and time delays. To fully realize the potential of CRISPR-based diagnostics, strategies for simplifying and streamlining sample preparation will be critical, especially in point-of-care settings when time and resources are limited [68].
6.2. Multiplexing
Multiplexing, or identifying numerous AMR indicators in a single experiment, is one of the most critical hurdles for CRISPR-based diagnostics. In clinical practice, infections are frequently caused by a combination of microorganisms, each of which may have unique resistance mechanisms. While multiplexed CRISPR assays are being developed, detecting a large range of resistance genes in a single test remains technically difficult [36]. Effective multiplexing without sacrificing sensitivity or accuracy, particularly in the case of mixed infections, will be critical for increasing the value of CRISPR-based diagnostics in clinical and public health contexts [36].
6.3. Regulatory Approval
CRISPR-based platforms, like any new diagnostic technology, must go through stringent regulatory approval processes to verify their safety, efficacy, and dependability. The regulatory approval procedures for CRISPR diagnostics are continually emerging, and these systems must meet high standards before being utilized in clinical practice. Obtaining regulatory authorization may take time, and negotiating the complicated regulatory landscape may postpone widespread implementation of CRISPR-based diagnostic tools [69].
6.4. Ethical and Privacy Concerns
The use of CRISPR technology in diagnostics poses numerous ethical and privacy concerns. One such risk is the possibility of misuse, particularly in the case of genetic data. The capacity to swiftly and efficiently edit or analyze genetic material can have serious ramifications for personal privacy, particularly when genetic data are stored and shared electronically. As CRISPR-based diagnostics become more extensively utilized, ethical concerns about permission, data privacy, and potential genetic prejudice must be addressed [70].
In the future, the integration of CRISPR diagnostics with other technologies such as microfluidics, artificial intelligence (AI), and biosensors may improve the capabilities of these platforms. Microfluidic devices may enable on-chip amplification and detection of genetic material, resulting in faster and more portable diagnostics. AI could help interpret diagnostic results and improve accuracy, particularly when dealing with complicated data sets or mixed illnesses [46,71]. Furthermore, ongoing innovation and surveillance will be required to keep up with the changing landscape of AMR, allowing CRISPR diagnostics to remain effective against growing resistance patterns and novel pathogens.
7. CONCLUSION
CRISPR-based diagnostic tools have transformed the detection of AMR by providing faster, more accurate, and cost-effective alternatives than conventional approaches. SHERLOCK, DETECTR, and CRISPR-based LFAs have all shown promising results in detecting AMR gene in pathogens such as MRSA, CRE, and VRE. These platforms have several advantages, including high sensitivity, portability, and the capacity to produce data within 30 min to an hour, making them ideal for use in point-of-care settings. Despite their potential, problems such as sample preparation complexity, multiplexing requirements, regulatory approval, and ethical issues about genetic data must be addressed. Future improvements in CRISPR diagnostics, particularly those involving microfluidics and AI, will improve detection capabilities and enable multiplexed identification of resistance genes. Continued innovation and surveillance will be critical in keeping up with emerging infections and resistance patterns, ensuring that CRISPR-based diagnostics are effective in combatting AMR.
8. ACKNOWLEDGMENTS
All authors acknowledge the encouragement and support rendered by their respective institutions.
9. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
10. FINANCIAL SUPPORT AND SPONSORSHIP
This study was funded by ICMR/DHR.
11. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
12. ETHICAL APPROVALS
As this is a review article based on previously published literature, no ethical approval or informed consent was required.
13. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
14. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
15. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. Antimicrobial resistance:A growing serious threat for global public health. Healthcare (Basel) 2023;11:1946. [CrossRef]
2. Ahmed SK, Hussein S, Qurbani K, Ibrahim RH, Fareeq A, Mahmood KA, et al. Antimicrobial resistance:Impacts, challenges, and future prospects. J Med Surg Public Health 2024;2:100081. [CrossRef]
3. Aijaz M, Ahmad M, Ansari MA, Ahmad S. Antimicrobial resistance in a globalized world:Current challenges and future perspectives. Int J Pharm Drug Des 2023;1:7-22.
4. Rajbhandari P, Maharjan S, Aryal S, Pradhan P, Prajapati S. Multidrug resistant (MDR) and extensively drug-resistant (XDR) gram negative bacteria at a tertiary care hospital, in Lalitpur, Nepal. J Patan Acad Health Sci 2024;11:16-23. [CrossRef]
5. Tang KW, Millar BC, Moore JE. Antimicrobial resistance (AMR). Br J Biomed Sci 2023;80:11387. [CrossRef]
6. Endale H, Mathewos M, Abdeta D. Potential causes of spread of antimicrobial resistance and preventive measures in one health perspective-a review. Infect Drug Resist 2023;16:7515-45. [CrossRef]
7. Darby EM, Trampari E, Siasat P, Gaya MS, Alav I, Webber MA, et al. Molecular mechanisms of antibiotic resistance revisited. Nat Rev Microbiol 2023;21:280-95. [CrossRef]
8. Rosas NC, Lithgow T. Targeting bacterial outer-membrane remodelling to impact antimicrobial drug resistance. Trends Microbiol 2022;30:544-52. [CrossRef]
9. Aljeldah MM. Antimicrobial resistance and its spread is a global threat. Antibiotics (Basel) 2022;11:1082. [CrossRef]
10. Ahmad S, Lohiya S, Taksande A, Meshram RJ, Varma A, Vagha K, et al. A Comprehensive review of innovative paradigms in microbial detection and antimicrobial resistance:Beyond traditional cultural methods. Cureus 2024;16:e61476. [CrossRef]
11. Martin-Loeches I, Pereira JG, Teoh TK, Barlow G, Dortet L, Carrol ED, et al. Molecular antimicrobial susceptibility testing in sepsis. Future Microbiol 2024;19:61-72. [CrossRef]
12. Banerjee R, Patel R. Molecular diagnostics for genotypic detection of antibiotic resistance:Current landscape and future directions. JAC Antimicrob Resist 2023;5:dlad018. [CrossRef]
13. Rahman MA, Uddin BM, Ratan ZA, Salman MN, Yeasmin MM, Mahjabin M. Prevalence and antimicrobial resistance pattern with associated gens of extended spectrum b-lactamase, carbapenemases and methicillin resistant Staphylococcus aureus producing organisms isolated from neonatal sepsis in Dhaka city of Bangladesh. Bangladesh J Med Microbiol 2024;18:114-9. [CrossRef]
14. Varol GI. Conventional methods and molecular approaches for detection of bacterial food pathogens. In:The Broad Spectrum of Biological Sciences. Gaziantep:Gaziantep Üniversitesi Yay?nlar?;2024. 211.
15. Gavina K, Franco LC, Khan H, Lavik JP, Relich RF. Molecular point-of-care devices for the diagnosis of infectious diseases in resource-limited settings-a review of the current landscape, technical challenges, and clinical impact. J Clin Virol 2023;169:105613. [CrossRef]
16. Ganger S, Harale G, Majumdar P. Clustered regularly interspaced short palindromic repeats/CRISPR-associated (CRISPR/Cas) systems:Discovery, structure, classification, and general mechanism. In:CRISPR/Cas-Mediated Genome Editing in Plants. Florida:Apple Academic Press;2023. 65-97. [CrossRef]
17. Sabri S, Mustofa MK, Fouad MT, Mukherjee S, Fakruddin M, Shishir MA. Comprehensive analysis of CRISPR-cas systems in microbial and their multifaceted applications. Microb Bioact 2023;7:1-11. [CrossRef]
18. Khurana A, Sayed N, Singh V, Khurana I, Allawadhi P, Rawat PS, et al. A comprehensive overview of CRISPR/Cas 9 technology and application thereof in drug discovery. J Cell Biochem 2022;123:1674-98. [CrossRef]
19. Hosen A, Nishat MN, Soaib MM, Sharkar OS, Sahabuddin M, Sharif IH, et al. A review:CRISPR cas system and the mechanism with an inhibition of binding of CRISPR Cas-9. Nano Select 2025;6:e202400009. [CrossRef]
20. Hillary VE, Ceasar SA. A review on the mechanism and applications of CRISPR/Cas9/Cas12/Cas13/Cas14 proteins utilized for genome engineering. Mol Biotechnol 2023;65:311-25. [CrossRef]
21. Saeed U, Insaf RA, Piracha ZZ, Tariq MN, Sohail A, Abbasi UA, et al. Crisis averted:A world united against the menace of multiple drug-resistant superbugs-pioneering anti-AMR vaccines, RNA interference, nanomedicine, CRISPR-based antimicrobials, bacteriophage therapies, and clinical artificial intelligence strategies to safeguard global antimicrobial arsenal. Front Microbiol 2023;14:1270018. [CrossRef]
22. Weng Z, You Z, Yang J, Mohammad N, Lin M, Wei Q, et al. CRISPR-cas biochemistry and CRISPR-based molecular diagnostics. Angew Chem Int Ed Engl 2023;62:e202214987. [CrossRef]
23. Lan H, Shu W, Jiang D, Yu L, Xu G. Cas-based bacterial detection:Recent advances and perspectives. Analyst 2024;149:1398-415. [CrossRef]
24. Puig-Serra P, Casado-Rosas MC, Martinez-Lage M, Olalla-Sastre B, Alonso-Yanez A, Torres-Ruiz R, et al. CRISPR approaches for the diagnosis of human diseases. Int J Mol Sci 2022;23:1757. [CrossRef]
25. Chambial P, Thakur N, Bhukya PL, Subbaiyan A, Kumar U. Frontiers in superbug management:Innovating approaches to combat antimicrobial resistance. Arch Microbiol 2025;207:60. [CrossRef]
26. Bharathkumar N, Sunil A, Meera P, Aksah S, Kannan M, Saravanan KM, et al. CRISPR/Cas-based modifications for therapeutic applications:A review. Mol Biotechnol 2022;64:355-72. [CrossRef]
27. Lou J, Wang B, Li J, Ni P, Jin Y, Chen S, et al. The CRISPR-cas system as a tool for diagnosing and treating infectious diseases. Mol Biol Rep 2022;49:11301-11. [CrossRef]
28. Wang H, Jia C, Li H, Yin R, Chen J, Li Y, et al. Paving the way for precise diagnostics of antimicrobial resistant bacteria. Front Mol Biosci 2022;9:976705. [CrossRef]
29. De Dieu Habimana J, Huang R, Muhoza B, Kalisa YN, Han X, Deng W, et al. Mechanistic insights of CRISPR/Cas nucleases for programmable targeting and early-stage diagnosis:A review. Biosens Bioelectron 2022;203:114033. [CrossRef]
30. Sen D, Mukhopadhyay P. Antimicrobial resistance (AMR) management using CRISPR-Cas based genome editing. Gene Genome Ed 2024;7:100031. [CrossRef]
31. Zhang X, Huang Z, Zhang Y, Wang W, Ye Z, Liang P, et al. Mitigating antibiotic resistance:The utilization of CRISPR technology in detection. Biosensors (Basel) 2024;14:633. [CrossRef]
32. Lee HY, Min YH, Lee DG, Lee KH, Kim J, Lee MK, et al. CRISPR/Cas12a collateral cleavage-driven transcription amplification for direct nucleic acid detection. Anal Chem 2024;96:12270-6. [CrossRef]
33. Jaybhaye SG, Chavhan RL, Hinge VR, Deshmukh AS, Kadam US. CRISPR-cas assisted diagnostics of plant viruses and challenges. Virology 2024;597:110160. [CrossRef]
34. Cherkaoui D. Harnessing State-of-the-art Diagnostic Technologies for Point-of-care Testing of Emerging and Neglected Tropical Diseases. (Doctoral dissertation, UCL (University College London);2022.
35. Zhang L, Jiang H, Zhu Z, Liu J, Li B. Integrating CRISPR/Cas within isothermal amplification for point-of-care assay of nucleic acid. Talanta 2022;243:123388. [CrossRef]
36. Bohara K, Parsaeimehr A, Bhattarai S. CRISPR-based diagnostic in aquaculture:Application, potential/opportunities, and limitations. SSRN Electron J 2024;1-28. doi:10.2139/ssrn.4815342 [CrossRef]
37. Cullot G, Amintas S, KarembéL, Prouzet-Mauléon V, Rébillard J, Boureau L, et al. Specific high-sensitivity enzymatic reporter UnLOCKing-mediated detection of oncogenic BCR:ABL1 and EGFR rearrangements. CRISPR J 2023;6:140-51. [CrossRef]
38. Di Carlo E, Sorrentino C. State of the art CRISPR-based strategies for cancer diagnostics and treatment. Biomark Res 2024;12:156. [CrossRef]
39. Taha BA, Ahmed NM, Talreja RK, Haider AJ, Al Mashhadany Y, Al-Jubouri Q, et al. Synergizing nanomaterials and artificial intelligence in advanced optical biosensors for precision antimicrobial resistance diagnosis. ACS Synth Biol 2024;13:1600-20. [CrossRef]
40. Zahra A, Shahid A, Shamim A, Khan SH, Arshad MI. The SHERLOCK platform:An insight into advances in viral disease diagnosis. Mol Biotechnol 2023;65:699-714. [CrossRef]
41. Kulkarni A, Tanga S, Karmakar A, Hota A, Maji B. CRISPR-based precision molecular diagnostics for disease detection and surveillance. ACS Appl Bio Mater 2023;6:3927-45. [CrossRef]
42. Thakku Venkateswaran SG. Modular CRISPR-diagnostics for Infectious Diseases. (Doctoral dissertation, Massachusetts Institute of Technology);9-2022.
43. Lee S, Nam D, Park JS, Kim S, Lee ES, Cha BS, et al. Highly efficient DNA reporter for CRISPR/Cas12a-based specific and sensitive biosensor. Biochip J 2022;16:463-70. [CrossRef]
44. Mohammad N, Katkam SS, Wei Q. A sensitive and nonoptical CRISPR detection mechanism by sizing double-stranded l DNA reporter. Angew Chem Int Ed Engl 2022;61:e202213920. [CrossRef]
45. Chen J, Chen Y, Huang L, Lin X, Chen H, Xiang W, et al. Trans-nuclease activity of Cas9 activated by DNA or RNA target binding. Nat Biotechnol 2024;43:558-68. [CrossRef]
46. Hassan YM, Mohamed AS, Hassan YM, El-Sayed WM. Recent developments and future directions in point-of-care next-generation CRISPR-based rapid diagnosis. Clin Exp Med 2025;25:33. [CrossRef]
47. Mohammad N, Katkam SS, Wei Q. Recent advances in CRISPR-based biosensors for point-of-care pathogen detection. CRISPR J 2022;5:500-16. [CrossRef]
48. Zhang C, Sun L, Wang D, Li Y, Zhang L, Wang L, et al. Advances in antimicrobial resistance testing. Adv Clin Chem 2022;111:1-68. [CrossRef]
49. Sánchez E, Ali Z, Islam T, Mahfouz M. A CRISPR-based lateral flow assay for plant genotyping and pathogen diagnostics. Plant Biotechnol J 2022;20:2418-29. [CrossRef]
50. Wang H, Wu Q, Zhou M, Li C, Yan C, Huang L, et al. Development of a CRISPR/Cas9-integrated lateral flow strip for rapid and accurate detection of Salmonella. Food Control 2022;142:109203. [CrossRef]
51. Son H. Harnessing CRISPR/cas systems for DNA and RNA detection:Principles, techniques, and challenges. Biosensors (Basel) 2024;14:460. [CrossRef]
52. Bhattacharjee G, Gohil N, Khambhati K, Gajjar D, Abusharha A, Singh V. A paper-based assay for detecting hypervirulent Klebsiella pnuemoniae using CRISPR-Cas13a system. Microchem J 2024;203:110931. [CrossRef]
53. Alsharksi AN, Sirekbasan S, Gürkök-Tan T, Mustapha A. From tradition to innovation:Diverse molecular techniques in the fight against infectious diseases. Diagnostics (Basel) 2024;14:2876. [CrossRef]
54. Ahmed MM, Kayode HH, Okesanya OJ, Ukoaka BM, Eshun G, Mourid MR, et al. CRISPR-cas systems in the fight against antimicrobial resistance:Current status, potentials, and future directions. Infect Drug Resist 2024;17:5229-45. [CrossRef]
55. Ates A, Tastan C, Ermertcan S. Precision genome editing unveils a breakthrough in reversing antibiotic resistance:CRISPR/Cas9 targeting of multi-drug resistance genes in methicillin-resistant Staphylococcus aureus. bioRxiv. 2024. [CrossRef]
56. Olatunji AO, Olaboye JA, Maha CC, Kolawole TO, Abdul S. Next-generation strategies to combat antimicrobial resistance:Integrating genomics, CRISPR, and novel therapeutics for effective treatment. Eng Sci Technol J 2024;5:2284-303. [CrossRef]
57. De la Fuente Tagarro C, Martín-González D, De Lucas A, Bordel S, Santos-Beneit F. Current knowledge on CRISPR strategies against antimicrobial-resistant bacteria. Antibiotics (Basel) 2024;13:1141. [CrossRef]
58. Dholpuria S, Anand S, Singh KS, Chaudhary S, Panwar H. Genomics innovations and advanced technologies. In:Biotechnological Interventions Augmenting Livestock Health and Production. Singapore:Springer Nature Singapore;2023. 151-69. [CrossRef]
59. Ho CS, Wong CT, Aung TT, Lakshminarayanan R, Mehta JS, Rauz S, et al. Antimicrobial resistance:A concise update. Lancet Microbe 2024;6:100947. [CrossRef]
60. Liu G, Thomsen LE, Olsen JE. Antimicrobial-induced horizontal transfer of antimicrobial resistance genes in bacteria:A mini-review. J Antimicrob Chemother 2022;77:556-67. [CrossRef]
61. Schinas G, Dimopoulos G, Akinosoglou K. Understanding and implementing diagnostic stewardship:A guide for resident physicians in the era of antimicrobial resistance. Microorganisms 2023;11:2214. [CrossRef]
62. Jang H, Song J, Kim S, Byun JH, Lee KG, Park KH, et al. ANCA:Artificial nucleic acid circuit with argonaute protein for one-step isothermal detection of antibiotic-resistant bacteria. Nat Commun 2023;14:8033. [CrossRef]
63. Agha AS, Al-Samydai A, Aburjai T. New frontiers in CRISPR:Addressing antimicrobial resistance with Cas9, Cas12, Cas13, and Cas14. Heliyon 2025;11:e42013. [CrossRef]
64. Shi L, Gu R, Long J, Duan G, Yang H. Application of CRISPR-cas-based technology for the identification of tuberculosis, drug discovery and vaccine development. Mol Biol Rep 2024;51:466. [CrossRef]
65. Pereira HS, Tagliaferri TL, de Oliveira Mendes TA. Enlarging the toolbox against antimicrobial resistance:Aptamers and CRISPR-Cas. Front Microbiol 2021;12:606360. [CrossRef]
66. Yamin D, Uskokovi?V, Wakil AM, Goni MD, Shamsuddin SH, Mustafa FH, et al. Current and future technologies for the detection of antibiotic-resistant bacteria. Diagnostics (Basel) 2023;13:3246. [CrossRef]
67. Ghouneimy A, Mahas A, Marsic T, Aman R, Mahfouz M. CRISPR-based diagnostics:Challenges and potential solutions toward point-of-care applications. ACS Synth Biol 2022;12:1-16. [CrossRef]
68. Ghorui A, Baksi S. CRISPR/Cas9 technology:Challenges and drawbacks. J Adv Zool 2023;44:1965-71. [CrossRef]
69. Anliker B, Childs L, Rau J, Renner M, Schüle S, Schuessler-Lenz M, et al. Regulatory considerations for clinical trial applications with CRISPR-based medicinal products. CRISPR J 2022;5:364-76. [CrossRef]
70. D'souza RF, Mathew M, Surapaneni KM. A scoping review on the ethical issues in the use of CRISPR-Cas9 in the creation of human disease models. J Clin Diagn Res 2023;17:1-8.
71. Ansari MA, Verma D, Hamizan MA, Mukherjee MD, Mohd-Naim NF, Ahmed MU. Trends in aptasensing and the enhancement of diagnostic efficiency and accuracy. ACS Synth Biol 2025;14:21-40. [CrossRef]