1. INTRODUCTION
Crude oil, being an indicator of ever-increasing demand for global industry [1,2], has been recognized as one of the primary sources of pollutants found in the terrestrial [3], atmospheric [4], and ocean ecosystems [5]. Since 2020, there were an average of 1.3 thousand metric tons of significant oil leaks caused by tanker events annually. Over 700 metric tons of oil were spilled in one of the reported oil disasters in 2023 [6]. As crude oil contains both aromatic and aliphatic hydrocarbons (HCs), it has a substantial negative influence on the quality of the air, water, and soil in India [7]. Since some of the HCs in crude oil mass are categorized as volatile chemicals, the negative impacts of crude oil pollution are not restricted to the impacted areas. Due to their low boiling temperatures, these chemicals have the potential to leak oil into the surrounding atmosphere and pose a health risk to the general public. These volatile compounds are a subset of more general gaseous molecules known as volatile organic compounds, which are known to have harmful effects on the environment and human health [8]. More than 8 million people died in 2018 as a result of fossil fuel pollution, according to new research from Harvard University in partnership with the University of Birmingham, the University of Leicester, and University College London. This is significantly higher than previous research indicating that air pollution from burning fossil fuels like coal and diesel was responsible for roughly 1 in 5 deaths globally [9]. The quality of the hydrocarbon content in the crude oil affects the degradability of the various hydrocarbon components. Due to the complexity of their structure, oil containing significant levels of high-molecular-weight compounds is challenging to be broken down biologically [10]. Surfactants are amphiphilic molecules, either synthetic or biological, that are essential for lowering the surface tension between immiscible fluids, such as water and oil, thereby facilitating the absorption of HCs and the emulsification process. But disadvantages of chemical surfactants, such as high cost, toxicity, and lack of biodegradability have brought attention to biosurfactants (BS) (derived from microbes) that can offer a more environmentally friendly option [11]. BS improve the breakdown of crude oil, are environmentally safe and can be utilized in bioremediation procedures. Even at low concentrations, they are effective, biodegradable, and non-toxic [12]. Emulsification activity, surface tension-reducing, and foaming qualities of BS finds their widespread uses in food, cosmetics, and pharmaceutical products, etc. Surface and interfacial activity, tolerance to physical factors such as pH, temperature and chemical factors such as ionic strength, biodegradability, low toxicity on metabolism, specificity to targets, biocompatibility, and digestibility, capacity to produce or disrupt emulsions are all exhibited by BS [13]. Various bacterial genera such as Acinetobacter, Arthrobacter, Bacillus, Enterobacter, Halomonas, Pseudomonas, Rhodococcus and numerous fungal genera, including Cephalosporium, Rhizopus, Paecilomyces, Alternaria, Mucor, Talaromyces, Gliocladium, Aspergillus, Fusarium, Rhodotolura, Cladosporium, Geotrichum, Penicillium, Torulopsis, and Pleurotus, have been uncovered through various research to be able to utilize crude oil as a source of carbon [14]. Although there has been an upsurge in research into the production of BS, industrial-scale production has not yet been accomplished because of the low yield and high production costs [15]. This makes it necessary to isolate new, high-yielding microbes that produce BS. Therefore, the screening and characterization of microorganisms with increased production of BS was the main focus of the current work. The objective of the present research was to find a unique and promising strain that may produce BS, which may be investigated further in the context of oil-contaminated environments and bioremediation.
2. MATERIALS AND METHODS
2.1. Enrichment of Collected Contaminated Soil and Water Samples
Crude oil-contaminated soil and water samples were obtained from petrol bunks in Bengaluru, India in airtight zip lock covers and were immediately processed in the microbiology laboratory of Kristu Jayanti College, Bengaluru, India. Air samples were directly taken by plate exposure method in polluted air environment such as parking lot and garage. Enrichment of soil and water samples was carried out in Bushnell Haas broth (BHB) medium by Nnamchi et al. [16] with 1% (v/v) naphthalene added as a carbon source before sterilization to promote the growth of naphthalene-degrading microorganisms. Soil and water sample (1%) was added into the medium and kept in a shaking incubator at 28°C for 7 days at 120 rpm.
2.2. Isolation and Identification of Naphthalene Degraders
Enriched samples were serially diluted and spread-plated on the Bushnell Hass agar (BHA) medium containing 1% (v/v) naphthalene. The plates were incubated at 28°C for 48 h. A zone of clearance around the colonies was indicative of efficient naphthalene degradation [16]. Isolates were tentatively identified up to the generic level based on colony morphology, staining reactions, and biochemical tests.
2.3. Qualitative Screening for BS Production
Primary screening for BS production was conducted in cetyl trimethyl ammonium bromide (CTAB) agar plate method and blood agar hemolysis [17]. Secondary screening tests for BS production, namely, oil spread method (OSM), drop collapse method, emulsification index (EI-24), and surface tension (ST) measurement were conducted [17]. Sodium dodecyl sulfate (SDS) was used as a control surfactant for secondary screening methods. Distilled water was used as a negative control.
2.4. Growth Studies of BS Producing Naphthalene Degraders
Each isolate was inoculated in a BHB medium with 1% (v/v) naphthalene as a carbon source. The inoculated tubes were incubated at 28°C for 48 h. Growth was measured by turbidometry at 600 nm and the growth curve was plotted [16]. Estimation of residual naphthalene by gravitimetric assay was carried as per the modified method by Pavithra et al. [18] Naphthalene degradation (%) was calculated using the formula:
 |
w1= weight of naphthalene added in the medium and w2 = weight of residual naphthalene
2.5. Molecular Analysis of Selected Isolates by 16Sr-DNA Sequencing
Pure cultures of selected isolates were sent to Barcode Biosciences, Bengaluru for 16Sr-RNA and 18SrRNA sequencing and identification of the isolates to species level.
2.6. BS Production by the Selected Isolates
BHB medium with 1.0% (v/v) naphthalene was used for BS production. BS extraction was performed using chloroform: methanol (2:1) extraction [19]. Dry weight of BS was determined as per Arora et al. [19].
2.7. Characterization of Crude BS
CTAB/Methylene-blue agar test for Rhamnolipid, Biuret test for Lipopeptide, and Phosphate test for Phospholipid [20-23] was performed to determine the type of BS obtained in crude form. Crude BS from cell-free supernatant were analyzed by thin layer chromatography (TLC) [24]. Chloroform: Methanol: Water (65:15:2, v/v) was used as a solvent system. Color developing reagents, namely, spraying of 0.2% ethanolic solution of ninhydrin (for lipopeptide with red-pinkish spots), heating at 110°C for 20 min after spray of 1% sulphuric acid (H2SO4) (for glycolipids with brown spots) and spraying of iodine vapors (for lipids), was used to visualize the type of BS. Phospholipids detection was carried out by the phospholipid-specific spray method of Goswami and Frey [25] and Paul et al. [26]. The EI-24 and surface tension of the crude BS were estimated against different substrates such as petrol, diesel, and kerosene [19]. Critical micelle concentration (CMC) is defined as the concentration of an amphiphilic component at which the formation of micelles is initiated in the solution at constant surface tension [27-28]. Different concentrations of the crude BS (10 mg/L to 100 mg/L) were prepared and surface tension was measured. The CMC was determined for each crude BS by plotting surface tension as a function of BS concentration and expressed as mg/mL. All the measurements were recorded in triplicates. SDS was used as the control surfactant in all experiments.
2.8. Structural Characterization of the Crude BS
The concentration of protein in crude BS was determined by Lowry’s method [29] with bovine serum albumin (BSA) as standard. Carbohydrate was estimated by the phenol-sulfuric acid procedure of Dubois [30] with D-glucose as a standard whereas lipid content was estimated by the method of Folch et al. [31]. Fourier transform infrared (FTIR) spectra of the BS were carried out at VIT University, Vellore, India. The wavelength used was in the range of 490 cm−1 with 2 mm/s scan speed on an FTIR system to determine the functional groups dominant in the samples.
Two of the most efficient crude BS samples were analyzed for fatty acid composition using gas chromatograph-mass spectroscopy [24]. The Clarus 680 GC was utilized for the analysis using a fused silica column loaded with Elite-5MS (5% biphenyl 95% dimethylpolysiloxane, 30 m × 0.25 mm ID × 250 μm df), and Helium as a carrier gas to separate the components at a steady flow rate of 1 mL/min. Throughout the chromatographic run, the injector temperature was maintained at 260°C. The oven temperature was as follows when the 1 μL BS extract sample was fed into the device: 60°C for 2 min; then 300°C at a rate of 10°C min−1; finally, 300°C, where it was maintained for 6 min. Conditions for the mass detector were as follows: Ion source temperature of 240°C, transfer line temperature of 240°C, ionization mode electron impact at 70 eV, scan period of 0.2 s, and scan interval of 0.1 s. The fragments were obtained from 40 to 600 Da. The spectrums of the components were compared with the database of the spectrum of known components stored in the Gas chromatography-mass spectrometry (GC-MS) NIST library [32].
2.9. Statistical Analysis
SPSS for Windows, Version 20 was used for the statistical evaluation. All analysis was represented as mean or mean ± standard deviation. Further, statistical correlations were calculated using Pearson’s correlation coefficient and Spearman rank correlation coefficient (P = 0.01, 2-tailed).
3. RESULTS
3.1. Enrichment, Isolation, and Identification of Naphthalene Degraders
Soil and water samples contaminated with oil were enriched with oil-degrading microorganisms in BHB medium. The air settling technique was used to isolate and enumerate microbes from the air. The enrichment was based on the ability of the microbes to utilize naphthalene as the sole carbon source. Aliquots of enriched samples were further plated on BHA medium yielding twenty-one isolates with halo zone formation on the agar medium marking them as naphthalene degraders. These isolates were given isolate codes and pure cultures were maintained in Nutrient Agar and sabouraud dextrose agar slants. All the isolates were studied for their colony morphology and staining reactions [Table 1]. Gram-positive rods were predominant followed by Gram-negative rods and fungi.
Table 1: Screening methods for biosurfactant production.
| Sl. no. | Source | Isolate code | Group | Gram morphology | LPCB | Primary screening | Secondary screening | ||
|---|---|---|---|---|---|---|---|---|---|
| Haemolysis | CTAB method | Drop collapse | Oil spread method | ||||||
| 1. | Soil | S1 | Bacteria | Gram positive rods | NA | α | ++ (9.8 mm) | - | + |
| 2. | Soil | S2 | Bacteria | Gram positive rods | NA | α | +++ (10 mm) | + | + |
| 3. | Soil | S3 | Bacteria | Gram negative rods | NA | - | - | - | - |
| 4. | Soil | S4 | Bacteria | Gram negative rods | NA | - | - | - | - |
| 5. | Soil | S5 | Bacteria | Gram negative rods | NA | β | +++++ (12 mm) | + | + |
| 6. | Water | W1 | Bacteria | Gram positive rods | NA | α | - | - | + |
| 7. | Water | W2 | Bacteria | Gram positive rods | NA | - | ++ (9.7 mm) | + | + |
| 8. | Water | W3 | Bacteria | Gram negative rods | NA | β | - | - | - |
| 9. | Water | W4 | Bacteria | Gram negative rods | NA | β | - | - | + |
| 10. | Water | W5 | Bacteria | Gram positive rods | NA | α | - | - | - |
| 11. | Water | W6 | Bacteria | Gram negative rods | NA | - | - | - | - |
| 12. | Water | W7 | Bacteria | Gram positive rods | NA | - | + (5 mm) | + | + |
| 13. | Air | A1 | Fungi | NA | Penicillium sp. | - | - | - | - |
| 14. | Air | A2 | Bacteria | Gram positive cocci | NA | - | - | + | + |
| 15. | Air | A3 | Bacteria | Gram positive cocci | NA | - | - | - | - |
| 16. | Air | A4 | Fungi | NA | Mucor | - | - | - | - |
| 17. | Air | A5 | Bacteria | Gram negative rods | NA | - | - | - | + |
| 18. | Air | A6 | Bacteria | Gram negative rods | NA | - | - | - | - |
| 19. | Air | A7 | Fungi | NA | Trichoderma sp. | - | - | - | + |
| 20. | Air | A8 | Bacteria | Gram positive cocci | NA | - | - | - | - |
| 21. | Air | A9 | Fungi | NA | Aspergillus niger | - | + (5.1 mm) | + | + |
LPCB: Lactophenol cotton blue,
NA: Not applicable.
3.2. Qualitative Screening for BS Production
Primary and Secondary screening for BS production was carried out. It was observed that many isolates able to degrade naphthalene did not yield positive results during the screening method [Table 1]. Primary screening comprised hemolytic activity and the CTAB agar method. Only few isolates namely, S1, S2, S5, W1, W3, W4 and W5 exhibited hemolytic activity whereas few isolates (S1, S2, S5, W2, W7, and A9) produced blue halo zones in CTAB method. Isolate S5 showed the highest CTAB zone diameter (12 mm), followed by S2 (10 mm). Secondary screening focused on the OSM, drop collapse test, emulsification index, and surface tension determination to quantify the viscosity of the particular culture after the incubation period reaches the maximum growth [Table 1]. Only 9 isolates showed efficient results in the secondary screening process. Comparative methods were performed to distinguish the BS producing bacteria and four isolates (S5, W2, W7, and A9) were affirmed as efficient BS producing colonies using emulsification index data obtained [Figure 1]. EI-24 of SDS was determined as 76%. The efficacy of a BS is evaluated based on its capacity to reduce the surface tension (ST) value of the medium. In the present study, isolates S5, W2, W7, and A9 exhibited efficient surface tension-reducing ability. SDS reduces the surface tension to 75.2 mNm-1 [Figure 2].
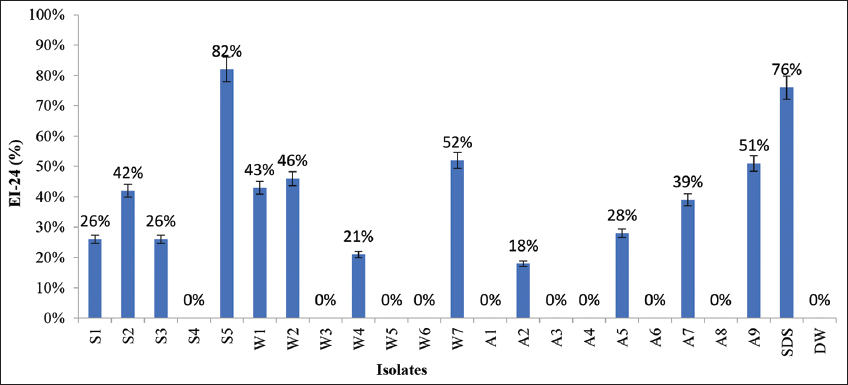 | Figure 1: Emulsification index (EI-24) of the isolates. SDS: Sodium dodecyl sulfate, DW: Distilled water. [Click here to view] |
 | Figure 2: Surface tension reduction by the isolates. SDS: Sodium dodecyl sulfate, DW: Distilled water. [Click here to view] |
3.3. Growth Studies of BS Producing Naphthalene Degraders
Isolate S5 and Isolate W2 showed the optimum growth and stability to produce the BS in the lab. In the gravimetric assay, the maximum percentage of naphthalene degradation was exhibited by Isolate W7 as 60% whereas the least amount of degradation was observed in Isolate W2 as 20% [Figure 3].
 | Figure 3: Gravitimetric assay of residual naphthalene. [Click here to view] |
3.4. Molecular Analysis of the Selected Isolates
Four of the best BS producers were identified by 16SrRNA and 18SrRNA sequencing and phylogenetic tree analysis as Pseudomonas aeruginosa (Isolate S5), Enterobacter mori (Isolate W2), Bacillus siamensis (Isolate W7), and Aspergillus niger (Isolate A9) [Table 2].
Table 2: Molecular characterization of selected isolates.
| Sl. no. | Isolate code | Identified as |
|---|---|---|
| 1. | S5 | Pseudomonas aeruginosa |
| 2. | W2 | Enterobacter mori |
| 3. | W7 | Bacillus siamensis |
| 4. | A9 | Aspergillus niger |
3.5. BS Production and Characterization
The highest crude BS yield was observed in P. aeruginosa (Isolate S5), with a production of 2.8 g/L, compared to 2.3 g/L by B. siamensis (Isolate W7), 2.1g/L by E. mori (Isolate W2), and 1.8 g/L by and A. niger (Isolate A9) [Table 3]. Qualitative analysis and TLC of the crude BS identified BS as phospholipid, rhamnolipid. lipopeptide and glycolipoprotein [Table 3]. Protein content was estimated to be highest in crude BS of A. niger (250 μg/mL) whereas carbohydrate content was highest in BS produced by E. mori and B. siamensis (64 μg/mL). Lipid content was highest in BS produced by A. niger [Table 4]. The crude BS produced was tested for emulsification efficiency against diesel, kerosene, and petrol. S5 recorded the highest EI-24 value of 73 ± 1.40% for diesel, followed by 65 ± 1.20% for kerosene and 45 ± 1.10% for petrol in comparison with the control surfactant [Figure 4]. Significant reduction in surface tension was observed in crude oil treated with BS produced by selected strains in comparison with the control surfactant [Figure 5]. The CMC of the extracted BS was also determined [Figure 6]. It was observed that the surface tension diminished with the increase in the concentration of the BS. Crude BS of B. siamensis reduced the surface tension to the least at 28.1 mNm−1 at 30 mg/L concentration, beyond which no decrease in surface tension was observed with increasing concentration of the BS. Therefore, the CMC of the crude BS of B. siamensis was determined as 30 mg/L. Similarly, CMS of the crude BS produced by P. aeruginosa, E. mori and A. niger was estimated as 50 mg/L, 60 mg/L and 40 mg/L respectively. The CMC of the SDS was determined as 30 mg/L that reduced the surface tension to 26.1 mNm-1. The result revealed that the crude BS by all the isolates possesses exquisite surface tension-reducing property with a lower CMC.
Table 3: Preliminary screening for types of biosurfactants.
| Sl. no. | Isolate code | Isolates | Dry weight of crude BS (g/L) (mean±SD) | Type of BS | Rf value (TLC) |
|---|---|---|---|---|---|
| 1. | S5 | Pseudomonas aeruginosa | 2.8±0.9d | + (Rhamnolipid) | 0.75 |
| 2. | W2 | Enterobacter mori | 2.1±0.5b | + (Sophorolipids) | 0.12 |
| 3. | W7 | Bacillus siamensis | 2.3±0.4c | + (Lipopeptides) | 0.81 |
| 4. | A9 | Aspergillus niger | 1.8±0.2a | + (Glycolipoprotein) | 0.51 |
Means sharing a common letter in the same column with soil types are not significantly different at P=0.05% level. TLC: Thin layer chromatography.
Table 4: Protein and carbohydrate content of the crude biosurfactant.
| Sl. no. | Isolates | Protein content (µg/mL) (Mean±SD) | Carbohydrate content (µg/mL) (Mean±SD) | Lipid content (µg/mL) (Mean±SD) |
|---|---|---|---|---|
| 1. | Pseudomonas aeruginosa (S5) | 50±1.2a | 62±0.9b | 33±0.9b |
| 2. | Enterobacter mori (W2) | 150±1.5b | 64±0.7c | 36±0.7c |
| 3. | Bacillus siamensis (W7) | 200±1.2c | 64±0.6c | 26±0.5a |
| 4. | Aspergillus niger (A9) | 250±1.1d | 44±0.5a | 60±0.7d |
Means sharing a common letter in the same column with soil types are not significantly different at P=0.05% level.
 | Figure 4: Emulsification index (EI-24) of the crude biosurfactant. [Click here to view] |
 | Figure 5: Surface tension reduction by the crude biosurfactant. [Click here to view] |
 | Figure 6: Critical micelle concentration of the crude biosurfactant produced by the isolates (P>0.05). [Click here to view] |
Molecular characterizations of the BS produced by the isolates were carried out by FTIR spectroscopy [Figures 7 and 8]. In FTIR spectra, detected peaks showed the presence of specific bonds characteristic of BS produced by many microorganisms. Peaks at 1,075 cm−1 indicated the amide groups of proteins. Strong bands between 3,000–3,600 cm−1 and 1,640–1,700 cm−1, as well as 1,500–1,620 cm−1, corresponded to NH group, C=O stretching in protein and NH bending in proteins, respectively, indicating proteins in the BS samples [Table 5].
 | Figure 7: Fourier transform infra red absorption spectra of biosurfactant produced by (a) Pseudomonas aeruginosa (Isolate S5) and (b) Enterobacter mori (Isolate W2). [Click here to view] |
 | Figure 8: Fourier transform infrared absorption spectra of biosurfactant generated by (a) Bacillus siamensis (Isolate W7) (b) Aspergillus niger (Isolate A9). [Click here to view] |
Table 5: Fourier transform infrared analysis of crude biosurfactant produced by the isolates.
| Sl. no. | Isolate | Group frequency wave number (Cm-1) | Functional group | Components |
|---|---|---|---|---|
| 1. | Pseudomonas aeruginosa (S5) | 3335.35 | O-H Stretch | Cellulose |
| 1640.36 | C=O and C=N Stretch | Peptide bond | ||
| 1020.18 | C-OH, C-O-C, C-C, Asymmetric stretching of P=O | Polysaccharides, Phosphodiesters | ||
| 2. | Enterobacter mori (W2) | 3335.35 | O-H Stretch | Cellulose |
| 1635.42 | C=O and C=N Stretch | Peptide bond | ||
| 1012.77 | C-OH, C-O-C, C-C, Asymmetric stretching of P=O | Polysaccharides Phosphodiesters | ||
| 3. | Bacillus subtilis (W7) | 3325.46 | O-H Stretching | Cellulose |
| 1637.89 | C=O and C=N Stretching | Peptide bond | ||
| 1064.65 | C-OH, C-O-C, C-C, Asymmetric stretching of P=O | Polysaccharides Phosphodiesters | ||
| 4. | Aspergillus niger (A9) | 3332.88 | O-H Stretching | Phenol |
| 1640.36 | C=O Stretching | Carbonyl/carboxyl group | ||
| 1020.18 | C-H Bending | Substituted Benzene |
GC-MS analysis of the crude BS produced by B. siamensis and A. niger revealed peaks characteristic of the BS [Figures 9 and 10]. Intense peaks were observed at a retention time 20.50 min for the crude BS produced by B. siamensis (W7) corresponding to hydroxyl octadecanoic acid showing successful incorporation of fatty acids into the sophorolipid side chain. For BS produced by A. niger, major peak compounds were identified at a retention time of 12.61, 20.25, and 27.54 min by comparing the data with the standard library as octacosane, 9-hydroxy-octadecanoic acid, and methyl linoleate.
 | Figure 9: Gas chromatography-mass spectrometry of biosurfactant produced by Bacillus siamensis (Isolate W7). [Click here to view] |
 | Figure 10: Gas chromatography-mass spectrometry of biosurfactant produced by Aspergillus niger (Isolate A9). [Click here to view] |
4. DISCUSSION
The study identified several microbial species capable of degrading pollutants into simpler, less toxic compounds. These microbes were sourced from contaminated environments and cultured in laboratory conditions. The findings from this study are consistent with several previous research works, supporting the capability of specific microbial strains to degrade a wide array of environmental pollutants. The microbial species identified in this study, particularly those from Pseudomonas, Bacillus, and Streptomyces, align with earlier reports by Bosch et al. [33], Subramanian and Menon [34] and Ferraro et al. [35] who also observed the degradation of HCs and heavy metals by these genera. Our study supports the idea that microbial communities with diverse enzymatic activities are essential for the effective bioremediation of polluted environments. The high petrol degradation efficiency of B. siamensis (60%) corresponds with observations by Li et al. [36], highlighting Bacillus species’ capacity for effective hydrocarbon breakdown.
Primary screening revealed hemolytic activity in 67% of the isolates. However, only 28.6% of these hemolytic isolates were confirmed as BS producers based on CTAB agar method results. Previous studies by Walter et al. [37] revealed BS producers with hemolytic activity, but all hemolytic species were not BS producers. The hemolysis test cannot be considered a reliable method since not all BSs have hemolytic activity and other compounds without surface activity may also cause hemolysis [38,39]. Despite this, the hemolysis test was used as the screening test of BS producers by Amiriyan et al. [40], Bicca et al. [41], and Tabatabaee et al. [42]. Hence, it is essential to regard hemolytic activity as an initial assessment and an unreliable indicator for detecting the presence of BS in a microbial culture.
In the present study, all the isolates did not exhibit positive results for all the tests. The drop collapse test was performed to check for BS production because most of the microorganisms produce extracellular or membrane-bound surface-active compounds essential to their survival [43]. These compounds can aid in nutrient transport, provide microbe-host interaction, or act as biocides. In emulsification tests, effective BSs are required to substantially decrease the oil-water interfacial tension to form an emulsion. Additionally, BSs should swiftly diffuse toward new interface.
Joshi et al. [44] reported that isolates capable of reducing the ST of the medium to >35 mN m−1 can be considered to be strong BS-producing microbes. Ghasemi et al. [45] reported that the measurement of surface tension (ST) is the most reliable method to screen BS-producing strains. In this study, we employed various screening methods to identify the most efficient BS producer. Numerous studies have established that the ability of a molecule to establish a stable emulsion doesn’t necessarily correlate with its effectiveness in reducing surface tension (ST). Thus, employing a combination of various screening methods might aid in selecting a BS that displays a range of characteristics, such as superior emulsification and wetting properties. Evaluating the results of screening tests can pose challenges, especially when dealing with a considerable number of strains being examined.
Biosurfactants can be produced within the cells as well as externally to act with the HCs in the environment so the growth might hinder in the process if the HCs are not available in the surroundings of the particular colonies. There was a clear correlation was noted between the production of BS (evidenced by a decline in the surface tension) and cell growth. This indicates that BS biosynthesis is linked to growth and is consistent with previous findings [19,46]. Residual naphthalene concentration measured was directly during the logarithmic phase of the cell cultures by Abalos et al. [47].
The characterization of BSs as phospholipids and lipopeptides in the present study is consistent with studies by Jamal et al. [48] and Ghasemi et al. [45], which documented similar BS classes in hydrocarbon-degrading microbes. Similar results were obtained previously for other LAB-derived BSs [49,50]. L. pentosus produced BS that was a blend of carbohydrate, lipid, and protein with a proportionate ratio of 1:3:6 [45]. TLC is established as one of the reliable techniques to detect BS [51]. In the present study, TLC analysis revealed the nature of BS that were consistent with studies by Marcelino et al. [52] and Sen et al. [53]. The observed EI-24 values for diesel, kerosene, and petrol align with Anuraj et al. [50], who demonstrated superior emulsification properties of BS against similar substrates. BS produced by L. plantarum CFR 2194 emulsified coconut oil (37.9%) and sunflower oil (19.43%) [31]. Significant reduction in surface tension by P. aeruginosa mirrors findings by Rashmi et al. [54], who reported a reduction in surface tension to 31 dyne/cm using similar substrates. BS produced by P. aeruginosa CGA1 reduced the surface tension of water from 72.1 mN/m to 35.0 ± 0.0 mN/m [55], whereas BS produced by Pseudomonas putida MTCC 2467 reduced surface tension of liquid to 35 mN/m [56]. Interestingly, much lower surface tension reduction of 28.8 mN/m was obtained by BS produced by Rhizopus arrhizus UCP1607 in low-cost culture medium [57]. The ability of a surfactant to reduce surface tension mainly depends on the CMC. CMC is the concentration at which the micelles start to form and the surface tension reaches its minimum value. An increase in surfactant concentration beyond CMC does not reduce the surface tension of the liquid any further. This is due to the fact that at CMC, surfactant molecules aggregate and form micelles in the polar or aqueous environment. Anaukwu et al. [55] observed the CMC at 60 mg/L for their strain P. aeruginosa CGA1. FTIR analysis confirmed the BS as phospholipids, rhamnolipids, lipopeptides, and glycolipoproteins. Strong absorption spectrum at 2,924.9593 cm–1 and 2,854.93 cm–1 indicates -CH2 and -CH3 bonds of hydrocarbon chains whereas a sharp peak at 1,709.48 cm–1 represented carbonyl groups of lipid moiety. The stretched peak at 1,215.10 cm-1 indicated C-O-C bond. Similar results were observed by Anaukwu et al. [55] and Jadhav et al. [58].
In our present study, octadecenoic acid, octasiloxane, and methyl linoleate were present with octadecanoic acid occurring majorily. The GC-MS analysis on BS by Anaukwu et al. [55] revealed the presence of fatty acid in the BS produced by CGA1 such as octadecanoic acid, cyclotetrasiloxane, cyclododecanol, methyl stearate, and tert-butyl isopropyl disulfide. Octadecanoic acid also known as stearic acid is a surfactant derived from natural fatty acids that possesses excellent surfactant properties and is biodegradable [59-61]. BS obtained from the isolate WG1 was identified as a glycolipid with long-chain fatty acids and polysaccharides fractions [62]. GC-MS data revealed the rhamnolipid nature of the BS produced by the P. aeruginosa SMVIT1 strain [63] and Pseudomonas spp. LM19 [64]. Sharma et al. [65] identified the fatty acid in biosurfactnat produced by Enterobacter faecium as hexadecanoic acid.
5. CONCLUSION
Microorganisms present in oil-polluted environments produce various bioactive compounds. Because of the diversity of their structures and functions, BS have garnered a lot of interest and attention. Bacillus and Pseudomonas species are among the common bacterial species that produce BS in these types of environments. Criteria for achieving the desired quantities and qualities of BS include enhanced purification processes and effective screening methodologies. With these concerns, twenty-one potential BS-producing isolates were obtained from petroleum-contaminated sites, and seven screening methods were employed for BS production. A strong negative correlation between SFT measurement and emulsification index in all the four isolates were that justifies the measurement as a BS producers’ screening tool. Once growing conditions for culture growth and BS production have been optimized, these isolates may be utilized for field studies in bioremediation and enhanced oil recovery. To speed up the bioremediation process, further research is required to isolate and identify different types of microorganisms in this area that can use long-chain HCs in a brief time. This study contributes to the growing body of evidence supporting the utility of microbial BS in environmental detoxification. Future work should focus on optimizing production conditions, scaling up processes, and exploring cost-effective substrates to improve commercial viability. The NMR technique is also used widely by researchers for identifying chemical structures found in BS. This spectroscopic technique determines the BS’ structure and establishes its purity and composition. The present research can be further directed to identify each component of the BS through NMR analysis of crude BS. BS could act as indicators in determining the germination index, which includes developing seeds and roots to assess BS’ toxic effects. Studies on BS application’ aspects of assessing its ecotoxicity on environments need to be investigated systematically.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. FUNDING
There is no funding to report.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
11. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
12. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Herrera AM, Hu L, Pastor D. Forecasting crude oil price volatility. Int J Forecast 2018;34:622-35.[CrossRef]
2. Kilian L, Murphy DP. The role of inventories and speculative trading in the global market for crude oil. J Appl Econ 2014;29:454-78.[CrossRef]
3. Rajabi H, Sharifipour M. Geotechnical properties of hydrocarbon-contaminated soils:A comprehensive review. Bull Eng Geol Environ 2019;78:3685-717.[CrossRef]
4. Afshar-Mohajer N, Li C, Rule AM, Katz J, Koehler K. A laboratory study of particulate and gaseous emissions from crude oil and crude oil-dispersant contaminated seawater due to breaking waves. Atmos Environ 2018;179:177-86.[CrossRef]
5. Afshar-Mohajer N, Fox MA, Koehler K. The human health risk estimation of inhaled oil spill emissions with and without adding dispersant. Sci Total Environ 2019;654:924-32.[CrossRef]
6. ITOPF. Global Oil Tanker Spills by Volume 1970-2023. Statista Research Department. England:ITOPF;2025.
7. Sihag S, Pathak H, Jaroli DP. Factors affecting the rate of biodegradation of polyaromatic hydrocarbons. Int J Pure Appl Biosci 2005;2:185-202.
8. Rajabi H, Mosleh MH, Mandal P, Lea-Langton A, Sedighi M. Emissions of volatile organic compounds from crude oil processing-Global emission inventory and environmental release. Sci Total Environ 2020;727:13?.[CrossRef]
9. Miller A. Fossil Fuel air Pollution Responsible for 1 in 5 Deaths Worldwide. United States:Harvard T.H. Chan School of Public Health;2021.
10. Zhang GL, Wu YT, Qian XP, Meng Q. Biodegradation of crude oil by Pseudomonas aeruginosa in the presence of rhamnolipids. J Zhejiang Univ Sci B 2005;6:725-30.[CrossRef]
11. Gallego JL, García-Martínez MJ, Llamas JF, Belloch C, Peláez AI, Sánchez J. Biodegradation of oil tank bottom sludge using microbial consortia. Biodegradation 2009;18:269-81.[CrossRef]
12. Das K, Mukherjee AK, Panigrahi S. Characterization of biosurfactant produced by a novel Pseudomonas aeruginosa strain for enhanced oil recovery. J Ind Microbiol Biotechnol 2008;35:799-808.
13. Latif MA, Ahmed T, Hossain MM. Bioremediation potential of a newly isolated Bacillus sp. petroleum hydrocarbon degradation. IOSR J Environ Sci Toxicol Food Technol 2011;5:55-60.
14. Sen R. Biotechnology in petroleum recovery:The microbial EOR. Prog Energy Combust Sci 2008;34:714-24.[CrossRef]
15. Franzetti A, Di Gennaro P, Bestetti G, Lasagni M, Pitea D, Collina E. Selection of surfactants for enhancing diesel hydrocarbons-contaminated media bioremediation. J Hazard Mater 2008;152:309-16.[CrossRef]
16. Nnamchi CI, Obeta JA, Ezeogu LI. Isolation and characterization of some polycyclic aromatic hydrocarbon degrading bacteria from Nsukka soils in Nigeria. Int J Environ Sci Technol 2006;3:181-90.[CrossRef]
17. Samanta SK, Singh OV, Jain RK. Polycyclic aromatic hydrocarbons:Environmental pollution and bioremediation. Trends Biotechnol 2002;20:243-8.[CrossRef]
18. Pavithra KG, Kumar PS, Jaikumar V, Rajan PS. Removal of colorants from wastewater:A review on sources and treatment strategies. J Ind Eng Chem 2019;75:1-19.[CrossRef]
19. Arora PK, Srivastava A, Singh VP. Bacterial degradation of nitrophenols and their derivatives. J Hazard Mater 2015;266:42-59.[CrossRef]
20. Siegmund I, Wagner F. New method for detecting rhamnolipids excreted by Pseudomonas species during growth on mineral agar. Biotechnol Tech 1991;5:265-8.[CrossRef]
21. Kayganich K, Murphy RC. Molecular species analysis of arachidonate containing glycerophosphocholines by tandem mass spectrometry. J Am Soc Mass Spectrom 1991;2:45-54.[CrossRef]
22. Feigner C, Besson F, Michel G. Studies on lipopeptide biosynthesis by Bacillus subtilis:Isolation and characterization of iturin-, surfactin+mutants. FEMS Microbiol Lett 1995;127:11-5.[CrossRef]
23. Okpokwasili GC, Ibiene AA. Enhancement of recovery of residual oil using a biosurfactant. Afr J Biotechnol 2006;5:453-6.
24. Aparna A, Srinikethan G, Smitha H. Production and characterization of biosurfactant produced by a novel Pseudomonas sp. 2B. Colloids Surf B Biointerfaces 2012;95:23-9.[CrossRef]
25. Goswami SK, Frey CF. Spray detection of phospholipids on thin-layer chromatograms. J Lipid Res 1971;12:509-10.[CrossRef]
26. Paul I, Mandal T, Mandal DD. Assessment of bacterial biosurfactant production and application in enhanced oil recovery (EOR) - A green approach. Environ Technol Innov 2022;28:102733.[CrossRef]
27. Pordeli H, Bloki Z, Azari AA, Kaveh K. Microbes and Infectious Diseases. Article-In-Press;2024.
28. Balakrishnan S, Arunagirinathan N, Rameshkumar MR, Indu P, Vijaykanth N, Almaary KS, et al. Molecular characterization of biosurfactant producing marine bacterium isolated from hydrocarbon-contaminated soil using 16S rRNA gene sequencing. J King Saud Univ Sci 2022;34:101871.[CrossRef]
29. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1992;193:265-75.[CrossRef]
30. Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem 1952;28:350-6.[CrossRef]
31. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497-509.[CrossRef]
32. Stein SE, Wallace W. NIST Libraries and Software. United States:U S Department of Commerce. 2023. 1-70.
33. Bosch MP, Rodríguez M, García-Valdés E. Capacity of different species of Pseudomonas to degrade naphthalene. Appl Microbiol Biotechnol 1988;29:250-3.
34. Subramanian A, Menon S. Novel polyaromatic hydrocarbon (PAH) degraders from oil contaminated soil samples. Int J Adv Res 2015;3:999-1006.
35. Ferraro A, Massini G, Miritana VM, Panico A, Pontoni L, Race M, et al. Bioaugmentation strategy to enhance polycyclic aromatic hydrocarbons anaerobic biodegradation in contaminated soils. Chemosphere 2021;275:130091.[CrossRef]
36. Li C, Cui C, Zhang J, Shen J, He B, Long Y, et al. Biodegradation of petroleum hydrocarbons based pollutants in contaminated soil by exogenous effective microorganisms and indigenous microbiome. Ecotoxicol Environ Saf 2023;253:114673.[CrossRef]
37. Walter V, Syldatk C, Hausmann R. Screening concepts for the isolation of biosurfactant producing microorganisms. In:Madame Curie Bioscience Database, Austin, TX:Landes Bioscience;2000-2013. Available from:https://www.ncbi.nlm.nih.gov/books/nbk6189 [Last accessed on 2024 Apr 17].
38. Safary A, Ardakani MR, Suraki AA, Khiavi MA, Motamedi H. Isolation and characterization of biosurfactant producing bacteria from Caspian Sea. Biotechnology 2010;9:378-82.[CrossRef]
39. Youssef NH, Duncan KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ. Comparison of methods to detect biosurfactant production by diverse microorganisms. J Microbiol Methods 2004;56:339-47.[CrossRef]
40. Amiriyan A, Mazaheri Assadi M, Saggadian VA, Noohi AA. Bioemulsifier production by Iranian oil reservoirs microorganisms. Iran J Environ Health Sci Eng 2004;1:28-35.
41. Bicca FC, Fleck LC, Ayub MA. Production of biosurfactant by hydrocarbon degrading Rhodococcus ruber and Rhodococcus erythropolis. Rev Microbiol 1999;30:231-6.[CrossRef]
42. Tabatabaee A, Assadi MM, Noohi AA, Sajadian VA. Isolation of biosurfactant producing bacteria from oil reservoirs. Iran J Environ Health Sci Eng 2005;2:6-12.
43. Tugrul T, Cansunar E. Detecting surfactant-producing microorganisms by the drop-collapse test. World J Microbiol Biotechnol 2005;21:851-3.[CrossRef]
44. Joshi S, Bharucha C, Desai AJ. Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20B. Bioresour Technol 2008;99:4603-8.[CrossRef]
45. Ghasemi A, Moosavi-Nasab M, Setoodeh P, Mesbahi G, Yousefi G. Biosurfactant production by lactic acid bacterium Pediococcus dextrinicus SHU1593 grown on different carbon sources:Strain screening followed by product characterization. Sci Rep 2019;9:5287.[CrossRef]
46. Pizzul L, Del Pilar Castillo M, Stenström J. Effect of rapeseed oil on the degradation of polycyclic aromatic hydrocarbons in soil by Rhodococcus wratislaviensis. Int Biodeterior Biodegradation 2007;59:111-8.[CrossRef]
47. Abalos A, Pinazo A, Infante MR, Casals M, García F, Manresa A. Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir 2001;17:1367-71.[CrossRef]
48. Jamal P, Alam MZ, Umi SZ, Wahab MA. Bioremediation of palm oil mill effluent using Aspergillus niger and Penicillium species. J Water Resour Protect 2011;3:524-30.
49. Marzieh D, Afzali D, Kermani M. Biosurfactant production for enhancing the solubility of phenanthrene in water by Pseudomonas putida. J Environ Health Sci Eng 2019;17:547-55.
50. Anuraj N, Sridharan S, Kumar M. Biosurfactant mediated remediation of phenanthrene contaminated soil by Bacillus licheniformis MTCC 5514. Biocatal Agric Biotechnol 2018;14:329-37.
51. Mousavi F, Beheshti-Maal K, Massah A. Production of sophorolipid from an identified current yeast, Lachancea thermotolerans BBMCZ7FA20, isolated from honey bee. Curr Microbiol 2015;71:303-10.[CrossRef]
52. Marcelino PR, Peres GF, Terán-Hilares R, Pagnocca FC, Rosa CA, Lacerda TM, et al. Biosurfactants production by yeasts using sugarcane bagasse hemicellulosic hydrolysate as new sustainable alternative for lignocellulosic biorefineries. Ind Crops Prod 2019;129:212-23.[CrossRef]
53. Sen S, Borah SN, Bora A, Deka S. Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microb Cell Fact 2017;16:95.[CrossRef]
54. Rashmi K, Rao AV, Alagawadi AR. Biodegradation of crude oil by Bacillus subtilis isolated from oil contaminated soil. Indian J Biotechnol 2011;10:378-84.
55. Anaukwu CG, Ogbukagu CM, Ekwealor IA. Optimized biosurfactant production by Pseudomonas aeruginosa strain CGA1 using agro-industrial waste as sole carbon source. Adv Microbiol 2020;10:543-62.[CrossRef]
56. Kanna R, Gummadi SN, Kumar GS. Production and characterization of biosurfactant by Pseudomonas putida MTCC 2467. J Biol Sci 2014;14:436-45.[CrossRef]
57. Pele MA, Ribeaux DR, Vieira ER, Souza AF, Luna MA, Rodriguez DM, et al. Conversion of renewable substrates for biosurfactant production by Rhizopus arrhizus UCP 1607 and enhancing the removal of diesel oil from marine soil. Electron J Biotechnol 2019;38:40-8.[CrossRef]
58. Jadhav SB, Surwase SN, Phugare SS, Jadhav JP. Response surface methodology mediated optimization of remazol orange decolorization in plain distilled water by Pseudomonas aeruginosa BCH. Int J Environ Sci Technol 2011;8:245-58.
59. Moussa LA, Ahmed ZA. Identification and characterization of biosurfactants produced by Rodococcus equi and Bacillus methlyotrophicus. J Biol Chem Environ Sci 2013;8:341-58.
60. Sharma D, Singh BS, Kapil S. Properties of biosurfactants. In:Biosurfactants of Lactic Acid Bacteria. Berlin:Springer;2016. 31-46.[CrossRef]
61. Parthipan P, Elumalai P, Sathishkumar K, Sabarinathan D, MuruganK, Benelli G, et al. Biosurfactant and enzyme mediated crude oil degradation by Pseudomonas stutzeri NA3 and Acinetobacter baumannii MN3. 3 Biotech 2017;7:278.[CrossRef]
62. Bhatia V, Singh BS. Isolation and partial structural &functional characterization of glycolipid biosurfactant producing Pichia sorbitophila WG1 from rotten grapes. J Chem Pharm Res 2016;8:357-67
63. Rath K, Singh AB, Chandan S, Vatsala RS. Isolation and characterization of a biosurfactant producing strain Pseudomonas aeruginosa SMVIT 1 from oil contaminated soil. J Sci Ind Res 2016;75:681-6.
64. Thio CW, Lim WH, Shah UK, Phang LY. Palm kernel fatty acid distillate as substrate for rhamnolipids production using Pseudomonas sp. LM19. Green Chem Lett Rev 2022;15:83-92.[CrossRef]
65. Sharma D, Saharan BS, Chauhan N, Procha S, Lal S. Isolation and functional characterization of novel biosurfactant produced by Enterococcus faecium. Springerplus 2015;4:4.[CrossRef]