1. INTRODUCTION
Rubia cordifolia L. (R. cordifolia) is an economically important plant known for its natural dyes and therapeutic properties. Certain compounds of R. cordifolia were shown to shorten plasma clotting time in vitro, indicating their procoagulant activity [1]. Also, several bioactivities, including anti-inflammatory, antioxidant, anti-platelet aggregation, antitumor, neuroprotective, anti-proliferative, immunomodulatory, and anti-cancer properties of R. cordifolia, have been reported [2, 3]. R. cordifolia compounds have DNA-binding properties, antifungal activity, and free radical inhibition. The Rubia genus, including R. cordifolia, produces anthraquinones (AQ) with various pharmacological effects [4].
The natural dyes (alizarin and purpurin) are extracted from R. cordifolia. AQs have been used in textile dyeing, pigment production, and cosmetic formulation for centuries due to their excellent colorfastness and vibrant colors [5]. Additionally, several studies revealed alizarin and purpurin’s anti-inflammatory, anti-cancer, antiviral, antimicrobial, and antioxidant properties, indicating pharmaceutical potential [4, 6]. Purpurin is more valuable, with its widespread use in the food and dye industries [7]. pH-sensitive qualities also enable purpurin to detect pH changes in solutions and living cells [8].
Hairy root cultures have demonstrated widespread effectiveness as an alternative production system for secondary metabolites from various plant species, owing to their genetic and biochemical stability, fast growth rate, and capacity to synthesize natural compounds at levels equivalent to those of plants grown in vivo [9, 10]. Numerous studies have documented the considerable efficacy of adventitious root tissues in metabolite productivity and biomass production [11, 12]. In a medium supplemented with phytohormones, adventitious roots exhibit vigorous growth and have showcased significant potential for accumulating valuable secondary metabolites [13, 14]. The synthesis of alizarin and purpurin occurs predominantly in the adventitious roots of R. cordifolia, providing a convenient target for optimizing their production through physiological and cultural approaches. However, limited studies have documented the production of alizarin and purpurin in the adventitious roots of R. cordifolia in vitro conditions [15, 4]. Chemical investigation of R. cordifolia roots led to the isolation of alizarin, purpurin, and other compounds [16, 17].
Furthermore, auxin influences the production of AQs relevant to alizarin/purpurin production, with mutant cells accumulating more secondary metabolites [18]. Supercritical fluid extraction using supercritical carbon dioxide has also been used to extract alizarin from Rubia tinctorum roots [19]. A study on the phenotypic and molecular variability in wild populations of R. cordifolia found variations in alizarin and purpurin content among different populations [16].
Previous research in other plants has shown that auxins can influence the production of secondary metabolites, including natural dyes, by regulating the expression of biosynthetic genes and metabolic pathways [20]. However, high auxin levels inhibit the accumulation of these secondary metabolites [21]. Elucidating the relationship between auxin concentration and alizarin/purpurin synthesis in R. cordifolia could enable enhanced dye yields and more sustainable production methods. Therefore, this study aimed to investigate the impacts of different auxin concentrations, pH levels, elicitors, and precursors on both adventitious root growth and the contents of alizarin and purpurin in R. cordifolia.
4. DISCUSSION
The multifaceted biological properties of AQ derivatives, encompassing antimicrobial, antileukemia, and antitumor activities, unfold a complex scientific panorama [24]. Adventitious root culture presents intricate challenges as an avant-garde avenue for secondary metabolite production [25]. These cultures, characterized by rapid growth and reliable metabolic productivity, serve as genuine hubs for biomass production. The selection of MS medium, known for its nutrient richness, introduces us to the idiosyncrasies of various plant species, each exhibiting unique preferences for medium strength [26].
Despite numerous biotechnological attempts to produce AQ through the hairy root cultures of R. cordifolia [27] and Rubia akane [28-30], the effects of different combinations of auxins, elicitors, and precursors remain largely unexplored.
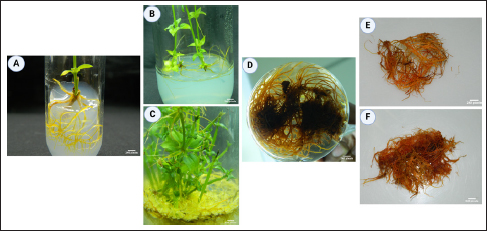
IBA, a common auxin used to promote adventitious root development in plant tissue culture [31-34], was effective in the current work. Notably, a low concentration of auxins alone proved sufficient for root development. The research indicates that the combination of IAA + IBA + NAA (1.5 + 0.5 + 0.75 mg/L) emerged as the optimal PGR for achieving the highest adventitious root induction and growth compared to media without PGR [Figure 1C; Table 1 and Supplementary Table 1]. In this context, the pivotal role of pericycle cells in root primordia formation is significant [35].
In adventitious root culture, the choice of medium composition plays a pivotal role, with MS medium being notable for its relatively high nutrient concentration. The optimal strength of MS medium varies widely across plant species, adding a layer of complexity to the establishment of standardized protocols. This study employed a full-strength MS medium to induce adventitious roots, revealing an intriguing interplay between nutrient strength, auxin concentrations, and plant responses. It is noteworthy that optimal MS medium strength can differ significantly among plant species. Rajesh et al. [36] and Lee and Paek [37] demonstrated the highest adventitious root biomass and bioactive compound production in Podophyllum hexandrum and Eleutherococcus koreanum, respectively, when utilizing 0.5× MS medium. Conversely, Cui et al. [38] reported variable responses in Hypericum perforatum, with adventitious root growth being enhanced at both 0.5× and 1× MS, while total phenolics increased specifically in 0.25× and 0.5× MS medium.
These divergent results suggest that the relationship between nutrient strength, adventitious root growth, and bioactive compound production is intricate and species-specific. Some studies have reported that the suppression of adventitious root growth and bioactive compound production under high nutrient strength may be attributed to osmotic stress or lipid peroxidation, intensifying water scarcity and toxicity [26]. Conversely, our investigation indicates that elevated nutrient strength enhances adventitious root induction. This disparity underscores the complexity of the factors influencing adventitious root culture outcomes, including auxin concentrations [Table 1 and Supplementary Table 1], plant species, and genotypes. Further investigation is required to understand the intricate relationship among these variables and uncover the mechanisms that regulate the ideal nutrient conditions for improved induction of adventitious roots and the production of bioactive compounds.
The increase in AQ levels resulting from the application of SA and L-Phe underscores their roles as signaling molecules, coordinating the synthesis of secondary metabolites. When recognized by plant receptors, elicitors such as SA and L-Phe activate a variety of effectors, including ion channels, NADPH oxidases, protein kinases, G proteins, and second messenger molecules like hydrogen jasmonic acid, peroxide, SA, and internal calcium release. This complex signaling cascade ultimately triggers upregulation of relevant genes, thereby stimulating the enhanced accumulation of secondary metabolites [39].
Plants respond quickly to abiotic stress by producing SA, activating the plant’s defense mechanism [40, 41]. The environmental and internal factors influencing the chorismate/succinyl benzoic acid biosynthesis pathway in the madder plant impact the synthesis of AQs [42].
Our research findings demonstrated that applying SA and L-Phe resulted in substantial alterations in accumulating secondary metabolites in madder adventitious roots. Interestingly, compared to the application of SA alone and the combination of SA and L-Phe, the presence of L-Phe alone led to a greater generation of bioactive substances [43]. These findings align with Mobin et al.’s (44) observations, who reported an elevated root growth index in Echinacea purpurea when L-Phe was added to root cultures at various concentrations. However, the effects of increased L-Phe concentrations on other plant species, such as strawberry and Larrea divaricata, exhibited variations, including suppressed cell growth and no discernible effect on root formation [45, 46]. These discrepancies may be attributed to variations in L-Phe concentrations, genotypes, and culture types [44, 47].
Notably, SA has been reported to inhibit cell proliferation in Salvia miltiorrhiza [48] and root growth in Bacopa monnieri [49]. However, our investigation indicates that SA favors the synthesis of bioactive substances in R. cordifolia [Table 2].
Consistent with our results, methyl jasmonate and SA significantly elevated anthraquinone accumulation in both transgenic and non-transgenic calluses of R. cordifolia [50]. L-Phe was found to enhance the root development of madder (Rubia tinctorum), while the effect of SA on root development parameters varied depending on its concentration. Interestingly, L-Phe did not notably impact the overall AQ content, encompassing both alizarin and purpurin. In contrast, SA led to an enhancement of AQs, with 20 µM SA proving the most optimal, resulting in the highest levels of total AQ, alizarin, and purpurin [43]. These findings highlight SA’s complex and context-dependent effects, emphasizing its dual role as a growth inhibitor and a promoter of secondary metabolite synthesis in R. cordifolia.
The influence of SA on plant growth has been multifaceted, as evidenced by its varying effects on Stemona plant root cultures. In the first week of culturing, root cultures were increased with 0.1 and 0.5 mM of SA, while a decrease occurred with 0.3 and 1.0 mM of SA. However, by the second week, root growth was reduced across all SA dosages [51]. The dynamic response underscores the complexity of SA’s impact on growth, emphasizing the role of several influencing factors, including SA concentration, culture type, genotype, culture duration, and the presence of elicitors [44, 46, 52].
Elicitors and precursors offer a strategic approach to augmenting the production of secondary metabolites, thereby enhancing their economic viability. Elicitors activate specific genes, triggering the biosynthesis of diverse chemical groups constituting secondary plant products [53, 54]. In our study, α-ketoglutaric acid (α-KGA) emerged as a pivotal factor influencing alizarin and purpurin production in R. cordifolia [Table 3]. α-Ketoglutaric acid is a significant intermediary in the Krebs cycle, which is crucial for cellular respiration and energy generation. It also serves as a precursor in the biosynthetic pathways leading to anthraquinone synthesis. The addition of α-ketoglutaric acid may accelerate the flux via metabolic pathways that lead to synthesizing secondary metabolites, including anthraquinones like alizarin and purpurin [55]. The isochorismate/O-succinyl benzoate route facilitated the conversion of carbon skeletons from shikimic acid and α-KGA into the A and B rings of AQ). Concurrently, core terpenoid blocks produced by the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway, specifically isopentenyl diphosphate and dimethyl allyl diphosphate, served as the source for the C ring [56]. Guo et al. [57] indicated that incorporating α-KGA into the medium speeds up the utilization of acetyl CoA, thereby affecting the metabolic flow of the MEP pathway.
Our investigation significantly impacted AQ production in adventitious root cultures by determining the distinct effects of elicitors and precursors [Table 3]. Comparable findings were reported in the adventitious roots of Morinda citrifolia L. [58]. Simultaneously, the co-application of elicitors inhibited both adventitious roots and AQ production in M. citrifolia [59]. The in vitro multiplication of adventitious roots unveils a promising avenue, serving as a reservoir for the cultivation of pharmaceutically significant AQ constituents, which underscores the potential of optimizing culture conditions to enhance the yield of valuable secondary metabolites with economic applications.
REFERENCES
1. Wang P, Wang J, El-Demerdash FM, Wei J. Coagulant compounds in Rubia cordifolia L. J Future Foods. 2023;3(3):220-4. [CrossRef].
2. Wen M, Chen Q, Chen W, Yang J, Zhou X, Zhang C, et al. A comprehensive review of Rubia cordifolia L.: Traditional uses, phytochemistry, pharmacological activities, and clinical applications. Front Pharmacol. 2022;13:965390. [CrossRef].
3. Humbare RB, Sarkar J, Kulkarni AA, Juwale MG, Deshmukh SH, Amalnerkar D, et al. 2022. Phytochemical characterization, antioxidant and anti-proliferative properties of Rubia cordifolia L. extracts prepared with improved extraction conditions. Antioxidants (Basel). 2022;11(5):1006. [CrossRef].
4. Murthy HN, Joseph KS, Paek KY, Park SY. Anthraquinone production from cell and organ cultures of Rubia species: An overview. Metabolites. 2022;13:39. [CrossRef].
5. Akar KB. Evaluation of alizarin and purpurin dyes for their ability to visualize latent fingermark on porous surfaces. Sci Justice. 2021;61:130-41. [CrossRef].
6. Tissier RC, Rigaud B, Thureau P, Huix-Rotllant M, Jaber M, Ferré N. Stressing the differences in alizarin and purpurin dyes through UV-visible light absorption and 1 H-NMR spectroscopies. Phys Chem Phys. 2022;24:19452-62. [CrossRef].
7. Mahanty S, Rathinasamy K, Suresh D. Spectral characterization of purpurin dye and its application in pH sensing, cell imaging and apoptosis detection. J Fluoresc. 2022;32:247-56. [CrossRef].
8. Maksimovic J, Cupic Ž, Manojlovic N, Deric A, Anic S, Kolar-Anic L. Bray–Liebhafsky oscillatory reaction as the matrix system for the kinetic determination of microquantities of alizarin and purpurin. React Kinet Mech Catal. 2020;130:655-68. [CrossRef].
9. Giri A, Narasu ML. Transgenic hairy roots: recent trends and applications. Biotechnol Adv. 2000;18:1-22.
10. Guillon S, Trémouillaux-Guiller J, Pati PK, Rideau M, Gantet P. Harnessing the potential of hairy roots: dawn of a new era. Trends Biotechnol. 2006;24:403-9.
11. Hahn EJ, Kim YS, Yu KW, Jeong CS, Paek KY. Adventitious root cultures of Panax ginseng CV Meyer and ginsenoside production through large-scale bioreactor system. J Plant Biotechnol. 2003;5:1-6.
12. Kim YS, Hahn EJ, Murthy HN, Paek KY. Adventitious root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol Lett. 2004;26:1619-22. [CrossRef].
13. Bourgaud F, Gravot A, Milesi S, Gontier E. Production of plant secondary metabolites: a historical perspective. Plant Sci. 2001;161:839-51. [CrossRef].
14. Murthy HN, Hahn EJ, Paek KY. Adventitious roots and secondary metabolism. Chin J Biotechnol. 2008;24:711-6. [CrossRef].
15. Mischenko NP, Fedoreyev SA, Glazunov VP, Chernoded GK, Bulgakov VP, Zhuravlev YN. Anthraquinone production by callus cultures of Rubia cordifolia. Fitoterapia. 1999;70:552-7.
16. Natarajan S, Mishra P, Vadivel M, Basha MG, Kumar A, Velusamy S. ISSR characterization and quantification of purpurin and alizarin in Rubia cordifolia L. populations from India. Biochem Genet. 2019;57:56-72. [CrossRef].
17. Chandrasekhar G, Shukla M, Kaul GKR, Chopra S, Pandey R. Characterization and antimicrobial evaluation of anthraquinones and triterpenes from Rubia cordifolia. J Asian Nat Prod Res. 2023;25:1110-6. [CrossRef].
18. Mariadoss A, Satdive R, Fulzele DP, Ramamoorthy S, Zayed H, Younes S, Rajasekaran C. Enhanced production of anthraquinones by gamma-irradiated cell cultures of Rubia cordifolia in a bioreactor. Ind Crops Prod. 2020;145:111987. [CrossRef].
19. Abou Elmaaty T, Sayed-Ahmed K, Magdi M, Elsisi H. An eco-friendly method of extracting alizarin from Rubia tinctorum roots under supercritical carbon dioxide and its application to wool dyeing. Sci Rep. 2023;13:30. [CrossRef].
20. Kim YC, Leveau J, McSpadden Gardener BB, Pierson EA, Pierson III LS, Ryu CM. The multifactorial basis for plant health promotion by plant-associated bacteria. Appl Environ Microbiol. 2011;77:1548-55. [CrossRef].
21. Baque MA, Hahn EJ, Paek KY. Growth, secondary metabolite production and antioxidant enzyme response of Morinda citrifolia adventitious root as affected by auxin and cytokinin. Plant Biotechnol Rep. 2010;4:109-16. [CrossRef].
22. Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473-97.
23. Schulte U, El-Shagi H, Zenk MH. Optimization of 19 Rubiaceae species in cell culture for the production of anthraquinones. Plant Cell Rep. 1984;3:51-4.
24. Tu HY, Huang AM, Teng CH, Hour TC, Yang SC, Pu YS, et al. Anthraquinone derivatives induce G2/M cell cycle arrest and apoptosis in NTUB1 cells. Bioorg Med Chem. 2011;19:5670-8. [CrossRef].
25. Carvalho EB, Curtis WR. Characterization of fluid-flow resistance in root cultures with a convective flow tubular bioreactor. Biotechnol Bioeng. 1998;60:375-84.
26. Cui HY, Abdullahil Baque M, Lee EJ, Paek KY. Scale-up of adventitious root cultures of Echinacea angustifolia in a pilot-scale bioreactor for the production of biomass and caffeic acid derivatives. Plant Biotechnol Rep. 2013;7:297-308. [CrossRef].
27. Kim YS, Shin SW. Production of anthraquinone derivatives by hairy roots of Rubia cordifolia var. pratensis. Korean J Pharmacogn. 1996;27:301-8.
28. Endo M, Sakata K, Katayama A. The pigments in the callus of Rubia akane and their dyeing properties. J Sericult Sci Jpn. 1997;66:107-12.
29. Mizutani H, Hashimoto O, Nakashima R, Nagai J. Anthraquinone production by cell suspension cultures of Rubia akane NAKAI. Biosci Biotechnol Biochem. 1997;61:1743-4.
30. Shim JJ, Shin JH, Pai T, Chung IS, Lee HJ. Permeabilization of elicited suspension culture of madder (Rubia akane Nakai) cells for release of anthraquinones. Biotechnol Tech. 1999;13:249-52.
31. Wynne J, McDonald MS. Adventitious root formation in woody plant tissue: the influence of light and indole-3-butyric acid (IBA) on adventitious root induction in Betula pendula. Vitro Cell Dev Biol Plant. 2002;38:210-2. [CrossRef].
32. Bae KH, Yun PY, Choi YE. Induction and in vitro proliferation of adventitious roots in Phyllanthus urinaria. Korean J Plant Res. 2009;22:454-60.
33. Wu CH, Dewir YH, Hahn EJ, Paek KY. Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol. 2006;49:193-9. [CrossRef].
34. Zhang J, Gao WY, Wang J, Li XL. Effects of sucrose concentration and exogenous hormones on growth and periplocin accumulation in adventitious roots of Periploca sepium Bunge. Acta Physiol Plant. 2012;34:1345-51. [CrossRef].
35. Kim YS, Hahn EJ, Yeung EC, Paek KY. Lateral root development and saponin accumulation as affected by IBA or NAA in adventitious root cultures of Panax ginseng CA Meyer. Vitro Cell Dev Biol Plant. 2003;39:245-9. [CrossRef].
36. Rajesh M, Sivanandhan G, Arun M, Vasudevan V, Theboral J. et al. Factors influencing podophyllotoxin production in adventitious root culture of Podophyllum hexandrum Royle. Acta Physiol Plant. 2014:36:1009-21. [CrossRef].
37. Lee EJ, Paek KY. Enhanced productivity of biomass and bioactive compounds through bioreactor cultures of Eleutherococcus koreanum Nakai adventitious roots affected by medium salt strength. Ind Crops Prod. 2012;36:460-5. [CrossRef].
38. Cui XH, Chakrabarty D, Lee EJ, Paek KY. Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresour Technol. 2010;101:4708-16. [CrossRef].
39. Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283-333. [CrossRef].
40. Senaratna T, Touchell D, Bunn E, Dixon K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000;30:157-61. [CrossRef].
41. Krantev A, Yordanova R, Janda T, Szalai G, Popova L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol. 2008;165:920-31. [CrossRef].
42. Orbán N, Boldizsár I, Szucs Z, Dános B. Influence of different elicitors on the synthesis of anthraquinone derivatives in Rubia tinctorum L. cell suspension cultures. Dyes Pigm. 2008;77:249-57. [CrossRef].
43. Demirci T, Aras Asci Ö, Göktürk Baydar N. Influence of salicylic acid and L-phenylalanine on the accumulation of anthraquinone and phenolic compounds in adventitious root cultures of madder (Rubia tinctorum L.). Plant Cell Tissue Organ Cult. 2021;144:313-24. [CrossRef].
44. Mobin M, Wu CH, Tewari RK, Paek KY. Studies on the glyphosate-induced amino acid starvation and addition of precursors on caffeic acid accumulation and profiles in adventitious roots of Echinacea purpurea (L.) Moench. Plant Cell Tissue Organ Cult. 2015;120:291-301. [CrossRef].
45. Edahiro JI, Nakamura M, Seki M, Furusaki S. Enhanced accumulation of anthocyanin in cultured strawberry cells by repetitive feeding of L-phenylalanine into the medium. J Biosci. 2005;99:43-47. [CrossRef].
46. Demirci T, Çelikkol Akçay U, Göktürk Baydar N. Effects of 24-epibrassinolide and l-phenylalanine on growth and caffeic acid derivative production in hairy root culture of Echinacea purpurea L. Moench. Acta Physiol Plant. 2020;42:1-11. [CrossRef].
47. Jacob A, Malpathak N. Manipulation of MS and B5 components for enhancement of growth and solasodine production in hairy root cultures of Solanum khasianum Clarke. Plant Cell Tissue Organ Cult. 2005;80:247-57. [CrossRef].
48. Dong J, Wan G, Liang Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotech. 2010;148:99-104. [CrossRef].
49. Largia MJV, Pothiraj G, Shilpha J, Ramesh M. Methyl jasmonate and salicylic acid synergism enhances bacoside A content in shoot cultures of Bacopa monnieri (L.). Plant Cell Tissue Organ Cult. 2015;122:9-20. [CrossRef].
50. Bulgakov VP, Tchernoded GK, Mischenko NP, Khodakovskaya MV, Glazunov VP, Radchenko SV, et al. Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J Biotech. 2002;97:213-21. [CrossRef].
51. Chaichana N, Dheeranupattana S. Effects of methyl jasmonate and salicylic acid on alkaloid production from in vitro culture of Stemona sp. Int J Biosci Biochem Bioinforma. 2012;2:146.
52. Govindaraju S, Arulselvi PI. Effect of cytokinin combined elicitors (l-phenylalanine, salicylic acid and chitosan) on in vitro propagation, secondary metabolites and molecular characterization of medicinal herb-Coleus aromaticus Benth (L). J Saudi Soc Agric Sci. 2018;17:435-44. [CrossRef].
53. Cosio EG, Frey T, Verduyn R, van Boom J, Ebel J. High-affinity binding of a synthetic heptaglucoside and fungal glucan phytoalexin elicitors to soybean membranes. FEBS Lett. 1990;271:223-6.
54. Menke FL, Parchmann S, Mueller MJ, Kijne JW, Memelink J. Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthetic genes in Catharanthus roseus. Plant Physiol. 1999;119:1289-96.
55. Chetri SK, Kapoor H, Agrawal V. Marked enhancement of sennoside bioactive compounds through precursor feeding in Cassia angustifolia Vahl and cloning of isochorismate synthase gene involved in its biosynthesis. Plant Cell Tissue Organ Cult. 2016;124:431-46.
56. Quevedo C, Perassolo M, Alechine E, Corach D, Giulietti AM, Talou JR. Increasing anthraquinone production by overexpression of 1-deoxy-D-xylulose-5-phosphate synthase in transgenic cell suspension cultures of Morinda citrifolia. Biotechnol Lett. 2010;32:997-1003. [CrossRef].
57. Guo ZG, Liu Y, Gong MZ, Chen W, Li WY. Regulation of vinblastine biosynthesis in cell suspension cultures of Catharanthus roseus. Plant Cell Tissue Organ Cult. 2013;112:43-54. [CrossRef].
58. Sreeranjini S, Siril EA. Optimising elicitors and precursors to enhance alizarin and purpurin production in adventitious roots of Morinda citrifolia L. Proc Natl Acad Sci India Sect B Biol Sci. 2015;85:725-31. [CrossRef].
59. Baque MA, Shiragi MHK, Lee EJ, Paek KY. Elicitor effect of chitosan and pectin on the biosynthesis of anthraquinones, phenolics and flavonoids in adventitious root suspension cultures of ‘Morinda citrifolia’ (L.). Aust J Crop Sci. 2012;6:1349-55.