1. INTRODUCTION
Anubias is an aquatic plant that is important to Thailand’s economy because it is beautiful and popular for use in aquariums, which has created demand in both domestic and export markets. Anubias spp. are monocotyledonous angiosperms in the family Araceae. The stem is an underwater rhizome. The leaves are ovate to lanceolate, with a dark green color. Anubias spp. grow well at temperatures of about 25°C at pH 6.5–7.4 [1]. It can be propagated by separating the shoots or dividing the rhizome and cultivating new shoots [2,3]. Thailand has been cultivating aquatic plants for export for 30 years. Some farms produce aquatic plants for domestic sales and export in many areas, such as Bangkok, Nakhon Pathom, Krabi, and Phuket. The volume and export value of aquatic plants in 2015 for Thailand was 9.7 million plants with worth 1.179 billion USD [4]. The top-five aquatic plants with high market demand and export value are Anubias, Nymphaea, Echinodorus, Hygrophila, and Cabomba [5]. It can be seen that Anubias is the number one in demand for aquatic plants. In 2018, Anubias had an export value of up to 13 million baht [6].
Anubias are extremely easy plants to grow and can survive a wide range of water parameters. Anubias minima has long and slender leaves in contrast to Anubias nana, which has shorter and rounder leaves. Like other Anubias plants, they can grow in both aquariums and terrariums, either submerged or emerged [1]. Experts estimate that the Thai aquatic plant market will continue to expand steadily and that Thailand’s competitiveness in the market will increase even more if there is promotion and support for the production of standard aquatic plants. Exotic beauty from plants native to this area is in demand in the market. This research aimed to combine gamma irradiation with tissue culture techniques to improve Anubias varieties by inducing mutation to create beautiful new morphological traits.
2. MATERIALS AND METHODS
2.1. Propagation of A. minima in Tissue Culture
A. minima plants were propagated in tissue culture on MS medium [7] with sugar 3% and gelrite 2.5 mg/L to create new shoots. When enough samples of A. minima were available for the experiment, the 8-week-old A. minima tissue was exposed to chronic gamma irradiation.
2.2. Chronic Gamma Irradiation
Tissue cultures of A. minima were exposed to chronic gamma radiation in a gamma room using a Cobalt-60 source at the Nuclear Technology Research Center, Faculty of Science, Kasetsart University. Different treatment groups of samples received total doses of 0, 26.58, 41.12, 65.31, 79.81, 103.81, or 127.91 Gy with a dose rate of 0.6 Gy/h. After irradiation, the plantlets were subcultured to a new MS medium for the M1V1 generation. At 60 days after irradiation, the number of surviving plantlets and the number of new shoots were recorded to calculate the 50% lethal dose (LD50) and 50% growth reduction (GR50) in comparison with the control (0 Gy).
2.3. Observing the Appearance of Leaf Mutations after Chronic Gamma Irradiation
After recording and calculating the initial data at the M1V1 generation, the surviving plants were again subcultured into new tissue culture vessels of MS medium for the M1V2 generation. They were observed for any morphological changes from the control, and new plantlets and new shoots from the M1V2 generation with changed morphology were separated to form the M1V3 generation. In the following generations, mutant plantlets were then screened and maintained separately from normal plantlets until it was found that the selected mutations were stable.
2.4. Chlorophyll Content Measurement on Leaf Mutants of A. minima
Chlorophyll content was determined in the leaves of mutated A. minima in the M1V10 generation, based on an adapted method described by Hipkins and Baker [8].
2.5. Experimental Design and Statistical Analysis
The experiment design was a completely randomized design with three replications. The data were analyzed using analysis of variance, after which means were compared using the least significant difference. The analyses were facilitated by the R program [9].
3. RESULTS AND DISCUSSION
3.1. Effect of Chronic Gamma Irradiation on Survival and Growth Rate in Tissue Culture of A. minima in the M1V1 Generation
After irradiation, the results showed that chronic gamma irradiation did not affect A. minima tissue survival. When data were statistically analyzed, there was no significant statistical difference [Table 1]. The LD50 could not be calculated because the irradiated sample had a high survival percentage of more than 50% [Figure 1]. This is similar to the experiments of Tangpong et al., Jompuk et al., and Sukin et al. [10-12], which reported that chronic gamma irradiation had no effect on the survival rate in the tissue culture of Anubias congensis N.E. Brown, Cryptocoryne wendtii “brown,” and A. nana.
Table 1: Survival percentage and growth rate percentage of Anubias minima in the M1V1 generation at 60 days after chronic gamma irradiation.
| Radiation dose (Gy) | Total number irradiated | Number of surviving plantlets | Survival percentage (% of control) | Number of new plantlets | Growth rate percentage (% of control) |
|---|---|---|---|---|---|
| 0 | 63 | 63 | 100.00 | 163 | 100.00a1 |
| 26.58 | 45 | 45 | 100.00 | 118 | 101.67a |
| 41.12 | 54 | 54 | 100.00 | 102 | 73.57b |
| 65.31 | 64 | 62 | 96.83 | 97 | 60.04c |
| 79.81 | 63 | 60 | 95.24 | 84 | 51.96cd |
| 103.81 | 48 | 48 | 100.00 | 76 | 61.23c |
| 127.91 | 51 | 51 | 100.00 | 63 | 48.45d |
| F-test | ns | ** | |||
| LSD0.05 | 5.08 | 9.44 | |||
| %CV | 3.00 | 7.60 | |||
Ns: Non-significant at 5% level.
** Significant at 1% level. 1means within columns followed by common superscript letters are not significantly different (P < 0.05)
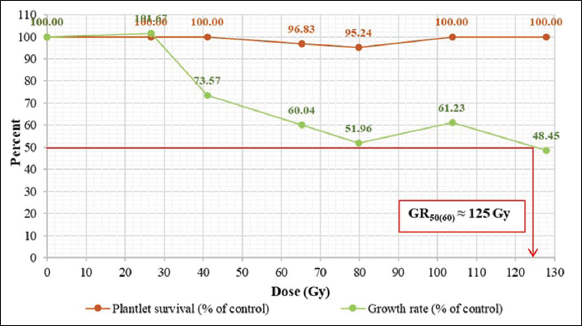 | Figure 1: Effects of radiation dose on survival percentage and growth rate percentage of Anubias minima in the M1V1 generation at 60 days after chronic irradiation. [Click here to view] |
Our study of the effect of chronic gamma irradiation on growth rate found that when the radiation dose increased, the growth rate percentage decreased. At the maximum radiation dose (127.91 Gy), the lowest growth rate was 48.45%. The statistical analysis showed that the irradiated samples had a significantly lower growth rate than the control [Table 1]. Therefore, the GR50 could be calculated as 125 Gy [Figure 1].
3.2. The Selected Leaf Mutants of A. minima after Chronic Gamma Irradiation
After irradiation, the survival and growth rate data were collected at the M1V1 generation. After that, the new plantlets were put into new bottles of MS medium and called the M1V2 generation. The morphological changes were observed in the new plantlets, and putative mutants were separated from the normal-looking plantlets. The results showed a variety of mutations, but the leaf mutation was the most apparent example. The mutants were screened up to the M1V10 generation. Four types of stable leaf mutations were found: Dark green leaves with light green specks, dark green leaves with white specks, light green leaves with dark green veins and specks, and white leaves with dark green veins and specks [Figure 2]. The most frequent leaf mutations were found at the dose of 79.81 Gy. This was similar to the findings of Tangpong et al. [10], which found that after chronic gamma irradiation, the most common mutation observed in the M1V3 generation of A. congensis was albinism. In the reports of chronic gamma irradiation on A. nana, the mutant characteristics in the M1V4 generation were variegated leaves, dwarfism, albinism, light green leaves, and abnormal leaves [12] and after chronic gamma irradiation, variation observed in the M1V2 generation of C. wendtii “brown” were small narrow leaves, green leaves, paler brown leaves, smaller leaves, dwarfism, and increased branching [11]. In the Kim et al. experiment, which observed changes in both Cymbidium hybrids’ leaf color and shape after gamma irradiation, the leaf color mutants showed the highest mutation frequency and spectrum [13].
 | Figure 2: Different types of leaf mutations in the M1V10 generation after irradiation: (a) Control (non-irradiation), (b) dark green leaves with light green specks, (c) dark green leaves with white specks, (d) light green leaves with dark green veins and specks, (e) white leaves with dark green veins and specks. [Click here to view] |
3.3. Chlorophyll Content Measurement on Leaf Mutants of A. minima
Fresh leaves from mutant A. minima leaves were used to determine the amount of chlorophyll content, and absorbance was measured using spectroscopy at 650 and 665 nm wavelengths. The measured values were used to calculate the content of chlorophyll A, chlorophyll B, and total chlorophyll.
The results showed that the chlorophyll content of four types of mutated leaves was different and lower than the control and decreased when the leaves were lighter in color. When statistically analyzed, there was a statistically significant difference. The leaves with the highest chlorophyll contents in the mutant leaf samples were dark green with light green specks, followed by dark green with white specks, light green with dark green veins and specks, and white with dark green veins and specks, respectively [Table 2]. Chlorophyll content can be used to estimate that the color of the leaves will change if the chlorophyll content varies. For example, mutant leaves with more dark green leaf surfaces than those with light green and white leaf surfaces had higher chlorophyll contents, which was the darker color of the solution [Figure 3]. In concordant research on gamma irradiation in Plantago ovata [14] and Curcuma hybrid “Laddawan,” it was found that gamma irradiation affected chlorophyll content, with irradiated samples having less chlorophyll content than the control (non-irradiated) [15]. In addition, Chanu et al. studied in tree tomato with gamma irradiation at doses of 0, 10, 25, 50, 75, and 100 Gy and reported that chlorophyll content decreases with the increase in gamma dose, and irradiated samples had less chlorophyll content than the control [16]. Yamaguchi, et al. reported that exposure to carbon ion beam, helium ion beam, and gamma rays induced albina, xantha, viridis, and other mutants such as striata (longitudinal white or yellow stripes) and maculate (green or yellow sports distributed over the leaf) [17].
Table 2: Chlorophyll content of leaf mutants of Anubias minima in M1V10 generation.
| Type of leaf mutant | Chlorophyll a (mg/g of fresh leaf weight) | Chlorophyll b (mg/g of fresh leaf weight) | Total chlorophyll (mg/g of fresh leaf weight) |
|---|---|---|---|
| Control | 2.308a | 1.179a | 3.520a1 |
| Dark green leaves with light green specks | 1.727b | 0.797b | 2.548b |
| Dark green leaves with white specks | 1.224c | 0.620c | 1.861c |
| Light green leaves with dark green veins and specks | 0.974d | 0.502cd | 1.490d |
| White leaves with dark green veins and specks | 0.753e | 0.381d | 1.145e |
| F-test | ** | ** | ** |
| LSD 0.05 | 0.206 | 0.144 | 0.069 |
| %CV | 8.12 | 11.43 | 1.79 |
** Significant at 1% level. 1means within columns followed by common superscript letters are not significantly different (P < 0.05)
 | Figure 3: The color of the solution extracted from leaf mutants of Anubias minima in the M1V10 generation: (a) Control, (b) dark green leaves with light green specks, (c) dark green leaves with white specks, (d) light green leaves with dark green veins and specks, (e) white leaves with dark green veins and specks. [Click here to view] |
4. CONCLUSION
The chronic gamma irradiation by cobalt-60 source can make leaf mutants of A. minima. The selected mutant plants can be transplanted with healthy and survive in water tanks.
5. ACKNOWLEDGMENTS
We would also like to thank the Nuclear Technology Research Center, Kasetsart University, for providing laboratory facilities and, equipment.
6. AUTHOR’S CONTRIBUTIONS
This work is part of the B.Sc. project of Mayuree Limtiyayotin (ML) advised by Peeranuch Jompuk (PJ). ML and PJ have designed the study, conducted the experimental work, and corrected the data. Choosak Jompuk (CJ) contributed to the data and statistical analysis. ML wrote and PJ and CJ revised the manuscript. Natnichaphu Sukin is the scientist who takes care of the instruments and tissue culture laboratory.
7. FUNDING
This project is funded by the National Research Council of Thailand (NRCT).
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Mühlberg H. The Complete Guide to Water Plants. United States:Sterling Publishing Co., Inc., German Democratic Republic;1982.
2. Rataj K, Horeman TJ. Aquarium Plant:Their Identification, Cultivation and Ecology. West Sulvania:T. F. H. Publ. Inc.;1977.
3. Cook CD. Aquatic Plant Book. 2nd revised edition. Amsterdam, New York:SPB Acadamic Publishing;1996.
4. Department of Agriculture, Agricultural Service Export Group. The Exporting Information of Aquatic Plants to Foreign Countries 2013-2015. Bangkok, Thailand:Bureau of Agricultural and Agricultural Materials Control;2015.
5. Thongkaemkaew P, Pojanasin C. Business analysis of aquatic plant production for export. Ramkhamhaeng Econ J 2016;2:1-17.
6. Rodloy A. Management Production and Marketing Beautiful Aquatic Plants in Thailand for Export and Sustainable use of Resources. Academic Document 2/2563. Freshwater Aquaculture Research and Development Division. Bangkok:Department of Fisheries;2020.
7. Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 1962;15:473-97. [CrossRef]
8. Hipkins MF, Baker NR. Photosynthetic Energy Transduction:A Practical Approach. Press, Arlington:IRL;1986.
9. Jompuk C. Statistics:Experimental Planning and Data Analysis in Plant Research with R Stat. Bangkok, Thailand:Kasetsart University Press;2012.
10. Tangpong P, Taychasinpitak T, Jompuk C, Jompuk P. Effect of acute and chronic gamma irradiations on in vitro culture of Anubias congensis N.E. Brown. Kasetsart J Nat Sci 2009;43:449-57.
11. Jompuk P, Jompuk C, Bunjongpetch A, Tangpong P. Effect of acute and chronic gamma irradiations on tissue culture of Cryptocoryne wendtii “brown“. Kasetsart J Nat Sci 2009;43:254-60.
12. Sukin N, Limtiyayothina M, Jompuk P. Inducing genetic diversity of Anubias nana using gamma rays. Agric Nat Resour 2020;54:85-90. [CrossRef]
13. Kim SH, Kim SW, Ahn JW, Ryu J, Kwon SJ, Kang BC, et al. Frequency, spectrum, and stability of leaf mutants induced by diverse γ-ray treatments in two Cymbidium hybrids. Plants (Basel) 2020;9:546. [CrossRef]
14. Saha P, Raychaudhuri SS, Chakraborty A, Sudarshan M. PIXE analysis of trace elements in relation to chlorophyll concentration in Plantago ovata Forsk. Appl Radiat Isot 2010;68:444-9. [CrossRef]
15. Tosri C, Chusreeaeom K, Limtiyayotin M, Sukin N, Jompuk P. Comparative effect of high energy electron beam and 137Cs gamma ray on survival, growth and chlorophyll content in curcuma hybrid “Laddawan“and determine proper dose for mutations breeding. Emirat J Food Agric 2019;31:321-7. [CrossRef]
16. Chanu AM, Thokchom R, Ningthoujam TH, Devi S, Meitei TR. Effect of gamma irradiation on the chlorophyll content of tree tomato (Solanum betaceum Cav.) in M1 generation. Pharma Innov J 2020;9:33-5.
17. Yamaguchi H, Hase Y, Tanaka A, Shikazono N, Degi K, Shimizu A, et al. Mutagenic effects of ion beam irradiation on rice. Breed Sci 2009;59:169-77. [CrossRef]