1. INTRODUCTION
Dyes or colorants are additives applied to food products or pharmaceutical preparations to impart or improve the color of a material. In the food production chain, food colorants play an essential role in covering unpleasant attributes and/or enhancing the natural properties of a food product [1,2]. Colorants are also added to pharmaceutical preparations to increase acceptability, maintain the stability of active ingredients, and as product identification during manufacturing and distribution [3,4]. Industries have used various synthetic dyes because they are stable, attractive in color, and inexpensive. However, there is a growing demand for natural colorants along with changes in consumer lifestyles, the raised awareness of artificial dyes’ side effects on health, and the emerging issues of environmental deterioration [1,2]. As a result, several natural dyes have been produced and approved for use in the United States, European Union, and Indonesia [1,5].
Anthocyanins are water-soluble pigments found especially in flowers, fruits, and roots or tubers that appear red in acidic environments, blue in alkaline conditions, or purple in neutral pH. Anthocyanins are categorized as a flavonoid even though they have a positively charged oxygen atom on the C ring of the basic flavonoid structure; hence, they are called flavylium ions (2-phenylchromenylium) [6,7].
Roselle (Hibiscus sabdariffa L.) is one of the plants rich in anthocyanins, including delphinidin-3-glucoside, cyanidin-3-glucoside, delphinidin-3-sambubioside, and cyanidin-3-sambubioside. These compounds are responsible for the red-to-purple color of the calyces [8,9]. In addition to their potential as colorants, they exhibit biological activities as antioxidant, anti-inflammatory, antibacterial, antitumor, antidiabetic, anti-hypertensive, and hepatoprotective agents [10]. For these reasons, anthocyanins in roselle have multiple functions as natural colorants and pharmacologically active ingredients. The food and pharmaceutical industries can explore this dual nature not only to create colorings but also to improve product characteristics (e.g., as preservatives) and functions (e.g., functional foods and pharmaceutical preparations with specific activities) [11].
A suitable extraction technology that is applicable on an industrial scale should, however, be developed first to utilize roselle calyces as a source of natural dyes. Several anthocyanin extraction methods from the calyces are heat-assisted extraction (HAE), ultrasound-assisted extraction (UAE), maceration, infusion, microwave-assisted extraction (MAE), and supercritical fluid extraction (SFE) [9,11-16]. Except for SFE, these methods are generally performed using water or water-ethanol mixtures as solvents [9]. UAE, MAE, and SFE are non-conventional extraction methods with several advantages, but the major drawback is the sizable investments in equipment.
Maceration is a conventional method most widely used by the public and industries. It is simple and economical, but the extraction efficiency is rather low. Several approaches have been introduced to improve it, such as heat-assisted and/or stirring-assisted maceration. However, since cold temperatures are more optimal for extracting anthocyanins [17], stirring-assisted extraction (SAE) is the better alternative to costly non-conventional extraction methods. Extraction efficiency depends on process variables, namely, solvent type, the ratio of crude drug weight to volume of solvent (S/L ratio), and extraction time. In addition to process variables, the optimal extraction conditions are also determined by the target compounds and the matrix of the plant material used. Therefore, the optimal SAE conditions should be determined to obtain roselle extracts with high anthocyanin contents.
Although the extraction of anthocyanins from roselle calyx has been studied in previous researches [12-14,18], this is the first work to optimize the SAE process and evaluate its efficiency against UAE. Therefore, the objectives of this research were threefold: To optimize the type of extraction solvent (50%, 70%, 95% ethanol, 50% ethanol acidified with citric acid, or 50% ethanol acidified with tartaric acid), extraction time (10, 15, or 20 min), and the S/L ratio (1:5, 1:10, or 1:15 g/mL) in the SAE of anthocyanins from roselle calyces. Four parameters were observed to evaluate the derived extracts: Color, pH, total anthocyanin content (mg/g) expressed as cyanidin-3-glucoside equivalent, and antioxidant activity measured with the DPPH radical scavenging method. This investigation provides relevant industries with preliminary data on the optimal SAE conditions on a laboratory scale — a stepping stone to further investigations for pilot and industrial-scale extraction.
2. MATERIALS AND METHODS
2.1. Plant Materials
Samples of purple roselle fruits [Figure 1] were harvested in Magetan Regency, East Java Province, Indonesia, in April 2021. These plant parts were authenticated by the Center for Traditional Medicine Information and Development, University of Surabaya, with a plant identification certificate number 1476/D.T/VI/2022. Picked samples were washed in clean tap water and drained. Then, calyces were removed from seed pods and air-dried. Dried calyces were later ground to powder using a blender (Philips HR 2222, Philips, Amsterdam, Netherlands) and passed through a 20-mesh sieve before being stored in a dark and airtight container.
 | Figure 1: Purple roselle parts: Fruits (a), seed pods (b), dry calyces (c), and dry calyx powder (d). [Click here to view] |
2.2. Selecting Extraction Methods
Before optimizing extraction variables, the optimal extraction method was selected from simple maceration (SM), SAE, and UAE. One gram of roselle calyx powder was added with 10 mL of 70% ethanol (Merck KGaA, Darmstadt, Germany). The mixture was then extracted with SM, SAE, or UAE for 15 min at room temperature. To perform SM, the mixed ingredients were soaked in a solvent in a beaker covered with aluminum foil. SAE was conducted the same way as SM but with stirring throughout the extraction time using a magnetic stirrer (Cimarec from Thermo Fisher Scientific, Waltham, MA, USA) at 200 rpm. UAE used an ultrasonic cleaner (Branson 1510 from Branson, Brookfield, Connecticut, USA) to sonicate the prepared mixture for 15 min with an ultrasonic power (P) of 150 W at 42 kHz. Afterward, extracts derived from each method were strained with a Whatman® Grade 1 qualitative filter paper (Merck) into a 10 mL volumetric flask and added with the proper solvent up to 10.0 mL. All the extracts were then observed organoleptically and further analyzed to determine their pH levels, total anthocyanin contents, and antioxidant activities.
2.3. Optimizing Extraction Solvents
The most favorable extraction method, a conclusion drawn from the previous research step, was further used to optimize the solvent. First, 1 g of roselle calyx powder was added with 50%, 70%, 95% ethanol, and 50% ethanol acidified with 2% citric acid (Merck KGaA), or 50% ethanol acidified with 0.75% tartaric acid (Merck KGaA) with the S/L ratio of 1:10 g/mL. Second, each mixture was extracted for 15 min at room temperature. Finally, the derived extracts were evaluated to determine their organoleptic properties, pH levels, total anthocyanin contents, and antioxidant activities.
2.4. Selecting Extraction Time
One gram of roselle calyx powder was extracted with the selected method and solvent, as ascertained in the previous two steps. With a fixed S/L ratio of 1:10 g/mL, the extraction was performed in three different durations (10, 15, and 20 min) at room temperature. Afterward, each of the derived extracts was observed organoleptically and chemically to determine pH levels, total anthocyanin contents, and antioxidant activities.
2.5. Selecting the Crude Drug-solvent Ratio
The next step to optimize extraction conditions was to find out the favorable ratio of crude drug weight to solvent volume. Roselle calyx powder was extracted with the optimized method, solvent, and time and a S/L ratio of 1:5, 1:10, or 1:15 g/mL. To create these ratios, crude drugs weighing 2.00, 1.00, and 0.67 g, respectively, were mixed with 10 mL of solvent before extraction.
2.6. Organoleptic Observations and pH Measurements
The organoleptic properties (color) of the extracts were observed visually with the naked eye, while the pH levels were measured using a pH meter (Mettler Toledo FP-20; Columbus, Ohio, US). Ten mL of the extract was placed in a 50 mL beaker glass. Then, the calibrated pH electrode was dipped in the extract, and the pH readings were recorded.
2.7. Measuring Total Anthocyanin Contents
Total anthocyanin contents were determined with the pH differential method introduced by Lee et al. with some modifications [19]. First, 250 μL of the extract was added with 5 mL of a pH 1.0 buffer solution. Second, another 250 μL of the same extract was drawn off using a pipette and then added with 5 mL of a pH 4.5 buffer solution. Afterward, the absorbance of both mixtures was read with a UV-Vis spectrophotometer (Shimadzu UV 1900 from Shimadzu, Kyoto, Japan) at wavelengths of 520 and 700 nm. Final absorbance (A) was calculated using the equation A = ([A520 − A700] pH1.0 – [A520 - A700] pH4.5). Total anthocyanin content, expressed as cyanidin-3-glucoside equivalents, was quantified using the equation below [19]:
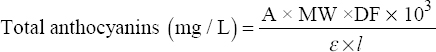 |
Where A = Final absorbance, MW = Molecular weight of cyanidin-3-glucoside (449.2 g/mol), DF = Dilution factor, ε = Molar extinction coefficient of cyanidin-3-glucoside (26,900 L × mol-1 × cm-1), l = Cuvette width (1 cm), and 103 = Conversion factor from g to mg. Total anthocyanin contents in mg/L were then converted into mg/g by considering the weight of the extracted crude drug and the volume of extract using the equation below:
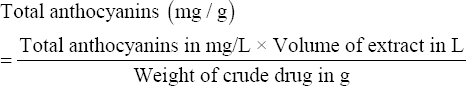 |
2.8. Measuring Antioxidant Activities
Free-radical scavenging method using DPPH was employed to screen the antioxidant activity of the derived extracts [20,21], with ascorbic acids as the reference compounds. The extract and the reference compound were each diluted until 5X concentrations. Then, 100 μL of the sample or reference compound solution was pipetted into a 96-well clear polystyrene microplate and added with 50 μL of DPPH (Sigma Aldrich Co., St. Louis, MO, USA) solution in ethanol (0.026%). The mixture was homogenized and then incubated in the dark for 10 min. The absorbance was read using a microplate reader (UVM 340 Biochrom from Biochrom Ltd., Cambridge, UK) at a wavelength of 517 nm. To create a blank sample, 100 μL of roselle extract with a proper concentration was added with 50 μL of ethanol. The absorbance values of the mixed DPPH solution (50 μL) added with ethanol (100 μL) was also recorded to obtain the absorbance of the control. The DPPH radical scavenging values were calculated using the equation below:
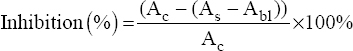 |
Where Ac = The absorbance of the control, As = The absorbance of the sample, and Abl = The absorbance of the blank sample. The sample’s potency in scavenging free radicals is expressed as the inhibitory concentration 50 (IC50) for DPPH. IC50 was calculated using the linear regression equation (y = bx + a) of the series of sample concentrations (x) versus percentages of inhibition (y).
2.9. TLC Fingerprinting of the Extracts
A sample of each extract was spotted on a TLC silica gel 60 F254 plate (Merck KGaA) with a 5 μL capillary tube (Camag, Muttenz, Switzerland). The mobile phase (from Merck KGaA) used was a combination of ethyl acetate, glacial acetic acid, formic acid, and demineralized water (100:11:11:26). The plate was eluted in a 10 × 10 cm twin-trough chamber (Camag) saturated with the mobile phase with a development distance of 8 cm. After elution, the plate was observed under white light, then derivatized with natural product-polyethylene glycol reagent (NP/PEG, from Merck KGaA) and further observed under 366 nm UV light (Camag).
2.10. Statistical Analysis
Depending on data distribution and variance, each extraction parameter was statistically analyzed with one-way ANOVA or Kruskal–Wallis (α = 0.05) to discover optimal extraction conditions. GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA, Windows Version 5.01) was used to aid the analysis.
3. RESULTS AND DISCUSSION
3.1. Comparison of SAE with SM and UAE
Organoleptically, roselle calyx extracts had a similar dark red color with a pH of 3.16–3.18 for all selected extraction methods [Figure 2]. However, SAE produced ones with the highest total anthocyanin content of 3.79 mg/g, which was significantly different from the UAE and SM outputs, 3.33 and 3.18 mg/g, respectively. Figure 3 presents the typical visible spectra of roselle calyx extracts at pH of 1.0 and 4.5.
 | Figure 2: Organoleptic properties and pH levels of roselle calyx extracts derived from simple maceration, stirring-assisted extraction, and ultrasound-assisted extraction. [Click here to view] |
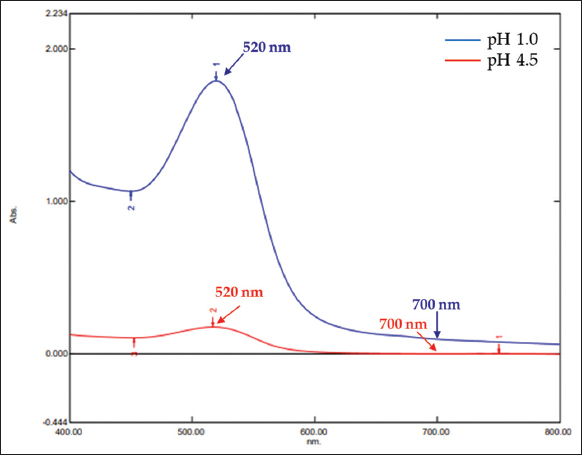 | Figure 3: Typical visible spectra of roselle extract at pH of 1.0 and 4.5. [Click here to view] |
These total anthocyanin contents corresponded to antioxidant activities in that SAE also created the most potent extract at low concentrations. As indicated by the IC50 values [Figure 4], the order of potency from highest to lowest was SAE (IC50 = 561 ppm), UAE (889 ppm), and SM (931 ppm). Therefore, SAE was chosen as the extraction method for the next stage of this research.
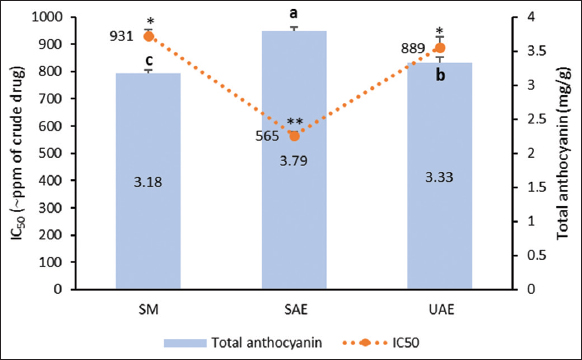 | Figure 4: Anthocyanin levels and antioxidant activities of roselle calyx extracts derived from simple maceration, stirring-assisted extraction, and ultrasound-assisted extraction. Data were obtained from the mean ± standard deviation of triplicate measurements (n = 3). Different notations or symbols on each graph indicate a significant difference among different extraction methods for the same response measured by least significant difference test (P ≤ 0.05). [Click here to view] |
Conventional extractions such as maceration, infusion, digestion, decoction, percolation, and Soxhlet extraction are currently the most popular methods used worldwide [22,23]. Maceration is a solid-liquid extraction that immerses plant parts/ingredients in a solvent for a predefined time. In a maceration, compounds are removed from within plant cells in three steps: penetration of solvent into cells, dissolution of compounds by solvent, and diffusion of miscella (solvent containing the said compounds) out of cells. The process efficiency can be improved in several ways, including stirring and increasing temperature. However, because anthocyanins are less stable in solutions at high temperatures [24], modifying the extraction method with stirring is the rational alternative. This research confirmed that SAE has better efficiency than the other conventional method (SM) and even the non-conventional method (UAE). This result matches previous research findings, where SAE proves more effective in extracting rosmarinic acid than conventional maceration (SM), heat reflux extraction (HRE), and MAE [25]. SAE has great potential to be used in industry, especially in the natural dye industry, because this extraction method has low instrumentation costs.
3.2. Effects of Different SAE Solvents on Roselle Calyx Extracts
Anthocyanin is a compound of the subclass flavonoids with good solubility in water and organic solvents such as methanol, ethanol, acetone, or mixtures thereof [6,23]. However, because methanol and acetone can lead to toxicity, a mixture of water and ethanol in various concentrations was used in this research. Roselle calyx extracts procured with 50% ethanol were dark red, while those with 70% and 95% ethanol were red and bright red. Extraction with 50% ethanol also created higher pH (3.01) than with 70% and 95% ethanol (2.84 and 2.7) [Figure 5].
 | Figure 5: Organoleptic properties and pH levels of roselle calyx extracts derived from stirring-assisted extraction with 50, 70, and 95% ethanol (EtOH) and the addition of citric acid and tartaric acid. [Click here to view] |
This trend was also seen in total anthocyanin contents [Figure 6]. Extraction with 50% ethanol produced higher anthocyanins than 70% and 95% ethanol, which were 3.48, 2.99, and 0.61 mg/g. These results are in accordance with the previous studies which conclude that the total anthocyanins extracted decrease with high ethanol concentrations [26]. Accordingly, 50% ethanol was the solvent of choice for optimized extraction. Adding acids to 50% ethanol can also improve extraction efficiency. Tartaric acid increased the total anthocyanin contents to 3.89 mg/g (11.78%), while citric acid elevated them to 4.33 mg/g (24.43%). Besides, extracts drawn with 50% ethanol acidified with tartaric acid and 70% ethanol exhibited the highest antioxidant activities, as evident in their respective IC50 values that were equivalent to 430 and 500 ppm of the crude drug. Meanwhile, those extracted with 95% ethanol showed the lowest antioxidant activities with an IC50 equivalent to 6989 ppm of the crude drug.
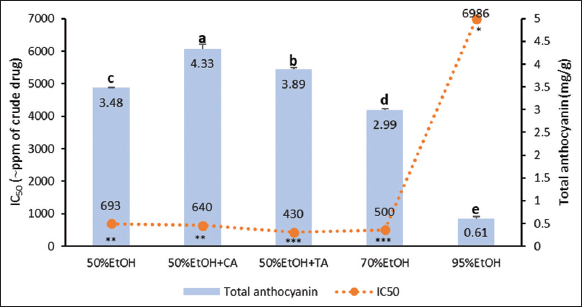 | Figure 6: Anthocyanin contents and antioxidant activities of roselle calyx extracts derived from stirring-assisted extraction with five different solvents. Data were obtained from the mean ± standard deviation of triplicate measurements (n = 3). Different notations or symbols on each graph indicate a significant difference among different solvents for the same response measured by least significant difference test (P ≤ 0.05). [Click here to view] |
In general, it can be inferred that a higher percentage of water in the water-ethanol solvent makes a more efficient extraction of anthocyanins. It is because the main anthocyanins in nature including roselle are glycosides with polar structure, namely, delphinidin-3-O-sambubioside and cyanidin-3-O-sambubioside [7, 27]. The addition of acids also increases efficiency. Anthocyanin forms flavylium cations at lower pH (pH ~ 3), making it far more soluble in water. Furthermore, an acidic environment keeps the flavylium ion stable and increases the intensity of anthocyanin’s red color [28]. The use of weak acids (e.g., acetic acid, citric acid, or formic acid) is more preferable, because the use of strong acids can cause destabilization of the anthocyanin molecules [23]. Other researchers have used solvents acidified with citric acid to increase the efficiency of anthocyanin extraction, both from roselle and other plant materials [26,29,30]. Therefore, 50% ethanol acidified with citric acid was chosen as the SAE solvent for the next steps of this study.
3.3. Effects of SAE Time Improvements on Roselle Calyx Extracts
Drawing out compounds from plant cells requires a sufficient time of contact between solvent and crude drug. Conventionally, maceration can take 1 h to 5 days to complete. A long extraction can, however, degrade particular compounds in extracts [22]. Therefore, SAE is expected to cut the required time, and for this reason, its efficiency in extracting anthocyanins in 10–20 min was evaluated. The results are presented in Figures 7 and 8.
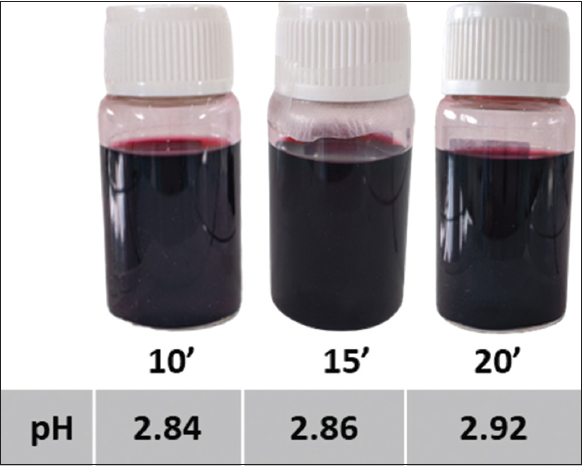 | Figure 7: Organoleptic properties and pH levels of roselle calyx extracts derived from stirring-assisted extraction with the solvent of 50% EtOH + citric acid for 10, 15, and 20 min. [Click here to view] |
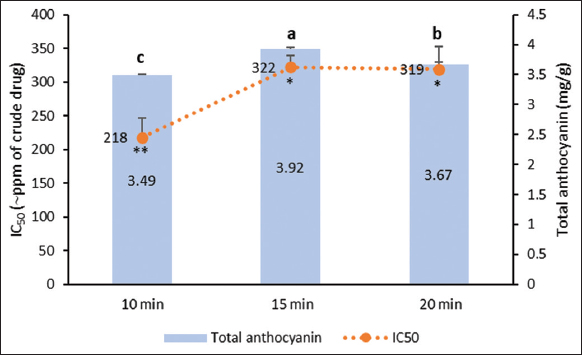 | Figure 8: Anthocyanin contents and antioxidant activities of roselle calyx extracts derived from 10 to 20 min of stirring-assisted extraction using 50% ethanol solvent acidified with citric acid. Data were obtained from the mean ± standard of triplicate measurements (n = 3). Different notations or symbols on each graph indicate a significant difference among different SAE time for the same response measured by least significant difference test (P ≤ 0.05). [Click here to view] |
Roselle calyx extracts from 10, 15, and 20 min of SAE had the same color, dark red, but the pH level tended to increase over the extraction time [Figure 7]. SAE conducted for 15 min produced anthocyanins higher than in shorter extraction time, 10 min. However, this trend did not continue to 20-min extraction because the total anthocyanin was lower. Anthocyanins are found in the vacuoles of plant cells. To bring them out from the plant cells, sufficient contact time between solvent and plant materials is required [23]. However, the long times of extraction could lead to the breakdown of the structures of sensitive compounds, such as cyanidin-3-O-glucoside [26].
On the contrary, the 10-min extraction gave the highest antioxidant activity [Figure 8]. Aside from anthocyanins, roselle also contains other phenolics, flavonoids, and organic acids acting as antioxidants [9]. These compounds were predicted to be extracted more in 10 min than 15 min. Because the 15-min extraction yielded the highest anthocyanin level, SAE in the next stage of this research was conducted in 15 min.
3.4. Effects of Ratios of Crude Drug Weight and Solvent Volume on Roselle Calyx Extracts
Effects of different S/L ratios (1:5, 1:10, 1:15 g/mL) in anthocyanin extraction on roselle calyx extracts are illustrated in Figures 9 and 10. There was no significant visual difference in extract color, and none was detected in the liquid pH levels [Figure 9]. Nevertheless, extraction with a smaller S/L ratio (1:15 g/mL) created a higher anthocyanin level and a more potent antioxidant [Figure 10].
 | Figure 9: Organoleptic properties and pH levels of roselle calyx extracts derived from 15-min stirring-assisted extraction using the solvent of 50% EtOH + citric acid and the S/L ratios of 1:5, 1:10, and 1:15 g/mL. [Click here to view] |
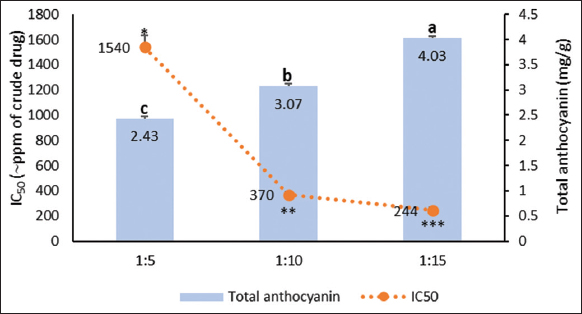 | Figure 10: Anthocyanin contents and antioxidant activities of roselle calyx extracts derived from 15-min stirring-assisted extraction using 50% ethanol solvent acidified with citric acid and the S/L ratios of 1:5, 1:10, and 1:15 g/mL. Data were obtained from the mean ± SD of triplicate measurements (n = 3). Different notations or symbols on each graph indicate a significant difference among different S/L ratio for the same response measured by least significant difference test (P ≤ 0.05). [Click here to view] |
A hypertonic environment needed for imparting colors will take form unless the S/L ratio is insufficient to fill up the crude drug. In this case, the compound will be kept inside the crude drug’s vacuole. However, if the crude drug-solvent combination reaches a certain ratio, plant cells will absorb the solvent rapidly and swell to the point of bursting, where the compound is simultaneously released inside the vacuole [13]. Therefore, the S/L ratio of 1:15 g/mL was chosen for the next step of extraction.
Crude drug characteristics also determine what S/L ratio is most favorable. For example, previous research found that relative to 1:5 and 1:10, the 1:2 ratio is optimal for extracting anthocyanins from fresh roselle calyces. However, 1:10 gives a better condition for dried calyces than 1:5 [14].
3.5. TLC Profile of Roselle Calyx Extracts
The dual functions of roselle calyx extracts, as colorant and bioactive agent, are determined by its major compound, namely, anthocyanins, phenolic acids, and flavonoids [10]. Therefore, the TLC profile of anthocyanins and phenolic acids was detected in this current research using cyanidin-3-O-glucoside (PhytoLab GmbH & Co. KG, Vestenbergsgreuth, Germany), chlorogenic acid (Sigma Aldrich Co.), and caffeic acid (Sigma Aldrich Co.) as reference compounds. TLC fingerprints of roselle calyx extracts showed that SAE with non-acidified ethanol solvents (tracks A-E) gave a similar anthocyanin pattern [red spots on Figure 11i], except for 95% ethanol (track E). Extraction with acidified ethanol solvents (tracks F-K) also shared a similar pattern: Poor separation of the anthocyanin compounds. In addition to anthocyanins, TLC fingerprinting also detected chlorogenic acid and caffeic acid in all the extracts, indicated by fluorescent bluish green spots [Figure 11ii].
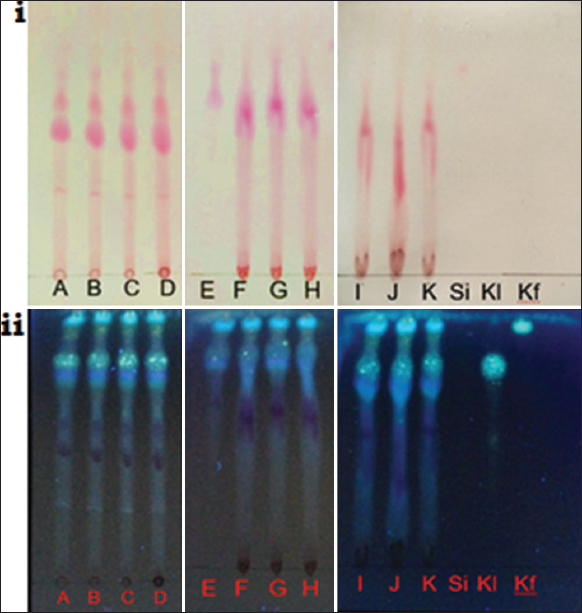 | Figure 11: Thin layer chromatography fingerprints of roselle calyx extracts (A-K) with three reference compounds: cyanidin-3-glucoside (Si), chlorogenic acid (Kl), and caffeic acid (Kf). Fingerprints are observed under visible light (i) and ultraviolet 366 nm after derivatization with NP/PEG (ii). Conditions of extraction (method, solvent, time, S/L ratio) for samples A-K are as follows SM, 70% EtOH, 15 min, 1:10 g/mL (A); stirring-assisted extraction (SAE), 70% EtOH, 15 min, 1:10 g/mL (B); UAE, 70% EtOH, 15 min, 1:10 g/mL (C); SAE, 50% EtOH, 15 min, 1:10 g/mL (D); SAE, 95% EtOH, 15 min, 1:10 g/mL (E); SAE, 50% EtOH + CA, 15 min, 1:10 g/mL (F); SAE, 50% EtOH + TA, 15 min, 1:10 g/mL (G); SAE, 50% EtOH + CA, 10 min, 1:10 g/mL (H); SAE, 50% EtOH + CA, 20 min, 1:10 g/mL (I); SAE, 50% EtOH + CA, 15 min, 1:5 g/mL (J); SAE, 50% EtOH + CA, 15 min, 1:15 g/mL (K). [Click here to view] |
The limitation of this research is that the extraction optimization was carried out on a small scale or laboratory scale. The optimal conditions obtained cannot be directly applied to industrial scale. Therefore, optimization of extraction on an increased scale needs to be performed further. SAE is proven to be easier to implement, lower investment, and more efficient than non-conventional method (UAE). To improve the results of this study, further research needs to be carried out to optimize other SAE variables, for example, stirring speed. Optimization on a larger scale, optimization of evaporation, and drying conditions of roselle extract are also worthy of further investigation.
4. CONCLUSION
In this study, conditions for extracting anthocyanins from roselle calyces have been optimized. The method of choice is SAE for 15 min using 50% ethanol solvent acidified with 2% citric acid and a S/L ratio of 1:15 g/mL. This conventional method proves more effective than SM and UAE. Furthermore, the optimal conditions identified in this study are suitable for extraction on a laboratory scale without sophisticated instrumentation, which can be scaled up for the mass production of natural colorants in the food and pharmaceutical industries. To address this need, further optimization of the extraction conditions on an increased scale is highly recommended.
5. ACKNOWLEDGMENTS
This research was funded by University of Surabaya with the grant number 013/SP-Lit/LPPM-01/FF/Int/FF/V/2022. The authors thank Dr. Nina Dewi Octaviyanti for the donations of cyanidin-3-O-glucoside.
6. AUTHORS’ CONTRIBUTIONS
Kartika Nurul Yulianda Setyawan conducted the data acquisition, data analysis, drafting manuscript, and technical support. Kartini Kartini conducted the research design, data and statistical analysis, drafting manuscript, critical revision of manuscript, funding the research, supervision, as well as final approval.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All data generated and analyzed are included within this research article.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Luzardo-Ocampo I, Ramírez-Jiménez AK, Yañez J, Mojica L, Luna-Vital DA. Technological applications of natural colorants in food systems:A review. Foods 2021;10:634. [CrossRef]
2. Sigurdson GT, Tang P, Giusti MM. Natural colorants:Food colorants from natural sources. Annu Rev Food Sci Technol 2017;8:261-80. [CrossRef]
3. Biswal PK, Mishar MK, Bhadouriya AS, Yadav VK. An updated review on colorants as the pharmaceutical excipients. Int J Pharm Chem Biol Sci 2015;5:1004-17.
4. Ramesh M, Muthuraman A. Flavoring and coloring agents:Health risks and potential problems. In:Grumezescu AM, Holban AM, editors. Natural and Artificial Flavoring Agents and Food Dyes. Cambridge:Academic Press;2018. 1-28. [CrossRef]
5. Health IMO. Regulation of the Ministry of Health of the Republic of Indonesia Number 033 Year 2012 Concerning Food Additional Materials;2012.
6. Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins:Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res 2017;61:1361779. [CrossRef]
7. Wallace TC, Giusti MM. Anthocyanins. Adv Nutr 2015;6:620-2. [CrossRef]
8. Borrás-Linares I, Fernández-Arroyo S, Arráez-Roman D, Palmeros-Suárez P, Del Val-Díaz R, Andrade-Gonzáles I, et al. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind Crops Prod 2015;69:385-94. [CrossRef]
9. Hapsari BW, Setyaningsih W. Methodologies in the analysis of phenolic compounds in roselle (Hibiscus sabdariffa L.):Composition, biological activity, and beneficial effects on human health. Horticulturae 2021;7:35. [CrossRef]
10. Izquierdo-Vega JA, Arteaga-Badillo DA, Sánchez-Gutiérrez M, Morales-González JA, Vargas-Mendoza N, Gómez-Aldapa CA, et al. Organic acids from Roselle (Hibiscus sabdariffa L.)- a brief review of its pharmacological effects. Biomedicines 2020;8:100. [CrossRef]
11. Jabeur I, Pereira E, Barros L, Calhelha RC, Sokovi?M, Oliveira MB, et al. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res Int 2017;100:717-23. [CrossRef]
12. Pinela J, Prieto M, Pereira E, Jabeur I, Barreiro MF, Barros L, et al. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem 2019;275:309-21. [CrossRef]
13. Pham TN, Nguyen TN, Lam TD, Tran TH, Nguyen DC, Vo DV, et al, editors. Effects of various solvent concentration, liquid-solid ratio, temperatures and time values on the extraction yield of anthocyanin from Vietnam Hibiscus sabdariffa L.(Roselle). IOP Conf Ser Mater Sci Eng 2019;542:012033. [CrossRef]
14. Chumsri P, Sirichote A, Itharat A. Studies on the optimum conditions for the extraction and concentration of roselle (Hibiscus sabdariffa Linn.) extract. Songklanakarin J Sci Technol 2008;30 Suppl 1:133-9.
15. Aryanti N, Nafiunisa A, Wardhani DH. Conventional and ultrasound-assisted extraction of anthocyanin from red and purple roselle (Hibiscus sabdari?a L.) calyces and characterisation of its anthocyanin powder. Int Food Res J 2019;26:529-35.
16. Wu HY, Yang KM, Chiang PY. Roselle anthocyanins:Antioxidant properties and stability to heat and pH. Molecules 2018;23:1357. [CrossRef]
17. Rasheed DM, Porzel A, Frolov A, El Seedi HR, Wessjohann LA, Farag MA. Comparative analysis of Hibiscus sabdariffa (Roselle) hot and cold extracts in respect to their potential for a-glucosidase inhibition. Food Chem 2018;250:236-44. [CrossRef]
18. Gartaula C, Karki DB. Optimization of extraction of anthocyanins from Roselle (Hibiscus sabdariffavar. Sabdariffa) in aqueous medium. J Food Sci Technol Nepal 2010;6:69-72. [CrossRef]
19. Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method:Collaborative study. J AOAC Int 2005;88:1269-78. [CrossRef]
20. Kartini K, Avanti C, Phechkrajang C, Vallisuta O. Antioxidant activity, HPTLC fingerprint and discriminant analysis of Plantago major leaves from diverse origins in Indonesia. Pharmacogn J 2019;11:1483-9. [CrossRef]
21. Sukweenadhi J, Yunita O, Setiawan F, Siagian MT, Avanti C. Antioxidant activity screening of seven Indonesian herbal extract. Biodiversitas 2020;21:2062-7. [CrossRef]
22. Cacique AP, Barbosa ÉS, de Pinho GP, Silvério FO. Maceration extraction conditions for determining the phenolic compounds and the antioxidant activity of Catharanthus roseus (L.) G. Don. Cienc Agrotecnologia 2020;44:e017420. [CrossRef]
23. Tena N, Asuero AG. Up-to-date analysis of the extraction methods for anthocyanins:Principles of the techniques, optimization, technical progress, and industrial application. Antioxidants (Basel) 2022;11:286. [CrossRef]
24. Mori K, Goto-Yamamoto N, Kitayama M, Hashizume K. Loss of anthocyanins in red-wine grape under high temperature. J Exp Bot 2007;58:1935-45. [CrossRef]
25. Sik B, HancznéEL, Kapcsándi V, Ajtony Z. Conventional and nonconventional extraction techniques for optimal extraction processes of rosmarinic acid from six Lamiaceae plants as determined by HPLC-DAD measurement. J Pharm Biomed Anal 2020;184:113173. [CrossRef]
26. Albuquerque BR, Pinela J, Barros L, Oliveira MB, Ferreira IC. Anthocyanin-rich extract of Jabuticaba epicarp as a natural colorant:Optimization of heat-and ultrasound-assisted extractions and application in a bakery product. Food Chem 2020;316:126364. [CrossRef]
27. Nakamura Y, Hidaka M, Masaki H, Seto H, Uozumi T. Major anthocyanin of the flowers of hibiscus (Hibiscus rosa-sinensis L.). Agric Biol Chem 1990;54:3345-6. [CrossRef]
28. Coutinho IB, Freitas A, Maçanita AL, Lima J. Effect of water content on the acid-base equilibrium of cyanidin-3-glucoside. Food Chem 2015;172:476-80. [CrossRef]
29. Pragalyaashree MM, Tiroutchelvame D, Sashikumar S. Degradation kinetics of anthocyanin extracted from Roselle calyces (Hibiscus sabdariffa). J Appl Pharm Sci 2018;8:57-63. [CrossRef]
30. Backes E, Pereira C, Barros L, Prieto M, Genena AK, Barreiro MF, et al. Recovery of bioactive anthocyanin pigments from Ficus carica L. Peel by heat, microwave, and ultrasound based extraction techniques. Food Res Int 2018;113:197-209. [CrossRef]