1. INTRODUCTION
Aquatic foods are important because of their role in food security and nutrition [1]. As it is one of the major sources of food fish supply for the growing population, despite the wide range of aquatic organisms, the major carp were cultured extensively which include Catla catla, Cirrhinus mrigala, and Labeo rohita. The composite culture of common carp, grass carp and silver carp, which are exotic, is also practiced. India leads in the production of Indian major carp followed by Bangladesh [2-4]. During the past six to seven decades, Cyprinus carpio (Common carp) has also been introduced into the aquaculture systems of India. The common carp is now one of the major contributors to the fisheries in the state of Uttar Pradesh [5]. Yet, the Indian major carp represent most of the inland fish landing in India.
Due to the irrational exploitation of finite natural sources, to date, most of the world’s demand for aquaculture was met with the aid of aquaculture farms. When cultured in a controlled environment, they will be subjected to an entirely different habitat type than that of natural. In such circumstances, what might be the impact of such habitat and diet changes on these Indian major carp needs to be studied. Fishes living in both wild and farmed environments exhibit not only morphological variances but also physiological changes and genetic variants, which may be a result of altered environmental conditions and dietary supplements. Despite a good number of reports on the above factors, there is a lack of literature stating the differential impact on the gut microbiome of the Indian carp.
The gut microbiome impacts the host fish with respect to the fish’s size, metabolism, feeding habits, and immunity [6]. As the fish gut microbiome influences the digestive physiology of the host, the habitat and dietary influence on the microbiota of the host need to be explored. The habitat effect on the gut microbiome of aquatic organisms has been investigated [7]. The anticipated advantage of this host-microbiota interaction or association can change if antibiotics modify the gut microbiome. The application of antibiotics has caused the evolution of antibiotic-resistant gut microbes in the aquaculture industry that may disseminate to other farms and animals including the human population [8]. Before two decades, most of the microbial works were based on conventional methods. Due to current advancements, next generation sequencing (NGS) has been entitled to identify both culturable and unculturable organisms [9]. Metagenomic studies have mostly concentrated on microbiota composition and host-microbiota interaction. The gut microbiome composition keeps varying and their origin is seldom studied. Therefore, studies on the factors affecting the gut microbiome are important. Further, the diversity and functional prediction of the uncultured bacteria is difficult to ascertain. Such questions can be answered using metagenomics and functional genomics. Metagenomics can aid to the determination of gut microbiome diversity by studying the hypervariable region of the 16s rDNA of prokaryotes [10]. Through this study, the species-specific difference in gut microbiome composition concerning the wild and farm-based environment has been explored with the aid of NGS.
2. MATERIALS AND METHODS
2.1. Sample Collection
For the present investigation, the fish were collected from Paithan, Aurangabad district, Maharashtra [Table 1]. C. catla (2 wild and 1 cultured), C. carpio (2 wild and 1 cultured), C. mrigala (1 wild and 1 cultured) and L. rohita (1 wild and 1 cultured) were investigated in the present study. Jayakwadi dam Nathsagar Jalashay (19°29’0.31”N, 75°22’24.63”E) was selected as the wild habitat. Cultured fish were procured from Fish seed Farm, Paithan (19°28’37.02”N, 75°22’6.07”E). Sterile Eppendorf tubes (1.5 mL) were used to collect the gut region of the carp. The tubes were transported in an ice-cold box. Before the DNA extraction, the samples were kept at −20°C.
Table 1: Sampling details for carps (culture ponds and wild).
| Serial number | Sample ID | Species | Habitat | Location from where the sample is collected |
|---|---|---|---|---|
| 1 | MF2 | L. rohita | Wild | Godawari, Jayakwadi |
| 2 | CATLAOLD | C. catla | Wild | Godawari, Jayakwadi |
| 3 | MF7 | C. catla | Wild | Godawari, Jayakwadi |
| 4 | MF1 | C. mrigala | Wild | Godawari, Jayakwadi |
| 5 | CC4 | Cyprinus carpio | Wild | Godawari, Jayakwadi |
| 6 | CYPRINUSOLD | C. carpio | Wild | Godawari, Jayakwadi |
| 7 | ROHU | L. rohita | Cultured | Paithan Fish Seed Farm |
| 8 | CATLA | C. catla | Cultured | Paithan Fish Seed Farm |
| 9 | MRIGAL | C. mrigala | Cultured | Paithan Fish Seed Farm |
| 10 | CYPRINUS | C. carpio | Cultured | Paithan Fish Seed Farm |
L. rohita: Labeo rohita, C. catla: Catla catla, C. mrigala: Cirrhinus mrigala, C. carpio: Cyprinus carpio
2.2. DNA Extraction
DNA was extracted from the sample (50 mg each) with the aid of the NucleoSpin DNA stool kit (MACHEREY-NAGEL, Germany) as per the protocol provided with the kit. Elution buffer (50 μL) was used to dissolve the extracted DNA. Till further processing, the extracted DNA samples were kept at −80°C.
2.3. Amplicon Sequencing
The DNA extracts were amplified using PCR for 25 cycles with hypervariable V3–V4 regions of the 16S rDNA gene [11]. The gene-specific sequences were supplemented with the nucleotide sequences used in Illumina adapter overhangs. The following primer sequences were used to target the region:
F 5’ TCGTCGGCAGCGTCA GATGTGTATAAGAGACAGCCTACG GGNGGCWGCAG3’
R5’GTCTCGTGGGCTCGGAGATGTGTATAAG AGACAGGACTACHVGGGTATCTAAT3’
The overhang adapter sequences that were added are mentioned below.
Forward overhang: 5’ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-[locus specific target primer]
Reverse overhang: 5’ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-[locus specific target primer]
A 1% agarose gel was used to visualize the amplicons. The library preparation was carried out using two-stage PCR. The successive cycles of PCR amplification were carried out as per the following details- denaturation (3 min at 95°C), 25 denaturation cycles (30 s at 95°C), annealing (30 s at 55°C), extension (30 s at 72°C) and a concluding extension (5 min at 72°C). After each PCR, PCR clean-up was done using AMPure XP Beads. Fluorometric quantification of the library was carried out through dsDNA binding dyes followed by the normalization. Before diluting the pooled library with a hybridization buffer, it was denatured using NaOH. After heat denaturation, the MiSeq v3 reagent kit was used to carry out Illumina MiSeq sequencing of the pooled library.
2.4. Sequence Analysis
To perform the sequence analysis, Quantitative Insights Into Microbial Ecology (QIIME2) software was employed [12]. Quality filtering of raw sequences was carried out. The sequence assignment to the samples was done based on barcodes. Primer sequences were removed. Chimeric sequences were detected. UCHIME [13] was used for the removal of these sequences. SILVA reference databases [14] were used for 16 s metagenomic analysis.
2.5. Statistical Analysis
Taxonomy-level reports in the output directory were created using QIIME 2. The taxonomic profiles at different levels were represented using MEGAN [15] and SILVA. The complexity of the species diversity was examined through Alpha diversity using Chao1, Faith’s Phylogenetic diversity, Shannon and Pielou’s evenness indices. The data was compiled into a spreadsheet. BioVinci data visualization package (Bioturing, San Diego, USA) was used to generate a violin plot. The beta diversity distance matrix was calculated from the OTU table and was presented in the form of an Unweighted Pair Group Method with Arithmetic mean (UPGMA) tree. Phylogenetic or count-based distance measurements were utilized to illustrate data similarities or differences using Principal Coordinates Analysis (PCoA). The analysis of the variations in each taxon’s relative abundance among the communities was done using Weighted UniFrac PCoA.
3. RESULTS AND DISCUSSION
3.1. Composition and Diversity in Gut Microbial Communities
In the present study, the total read count observed was 46,987, with the maximum and minimum reads of 8607 and 1383 respectively. In precise, 826 OTUs were assigned to various taxonomic levels. The taxonomic levels assigned included 18 phyla, 24 classes, 59 orders, 91 families, and 118 genera. Firmicutes, Proteobacteria and Cyanobacteria represented the three most dominant phyla in all carp species studied. Among the classified reads, Phylum Firmicutes represented in the range of 0–100%, Proteobacteria from 0% to 85%, and Cyanobacteria from 0% to 63%. Relative abundance in every sample at the phylum level is presented in Figure 1. Earlier reports have also highlighted a higher abundance of these phyla in the fish gut microbiome of Labeo rohita, Hypophthalmichthys molitrix, Aristichthys nobilis, Ctenopharyngodon idellus, Oncorhynchus mykiss, and Carassius auratus. Similar observations have been made in recent studies on grass carp, crucian carp, Rohu and Tilapia [10,16-21]. Firmicutes were predominant in the gut of cultured and wild Catla. The presence of Proteobacteria was variable in the species studied. Cyanobacteria were predominant in the gut of Rohu. Species-specific differences in gut microbiome were also noted. Some microbial phyla were species-specific which included Acidobacteria, Fibrobacteria and Nitrospirae in Catla; Gemmatimonadetes and Nanoarchaeota in the common carp.
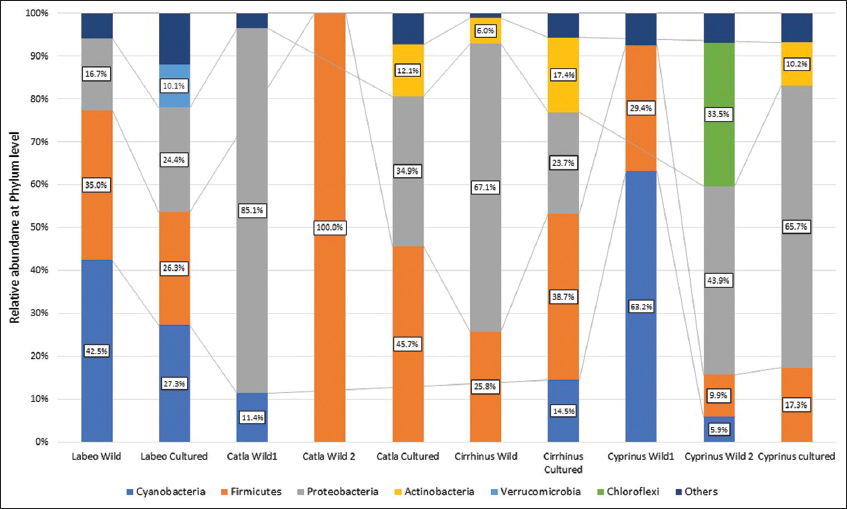 | Figure 1: Relative abundance of phyla in wild and cultured carps of 4 groups. [Click here to view] |
The phylum Proteobacteria is involved in the metabolism of nutrients in fish and accounts for the major proportion of the gut of aquatic animals [22]. The bacteria from the phylum Proteobacteria have a key role in carbon, nitrogen and sulphur cycling from sludge and municipal wastes [20,23]. Some Proteobacteria contribute to digestive physiology through enzyme secretion while some play role in the biosynthesis of riboflavin and biotin [24]. Firmicutes which are important for the metabolism of carbohydrates in man [25], dominated the gut of wild as well cultured forms. The Firmicutes also affect lipid metabolism, produce digestive enzymes, promote host metabolism, and increase the bioavailability of fatty acids [26]. A higher relative abundance of Firmicutes was seen in the cultured forms and unlike Bacteroidetes. The faster growth rate of the cultured forms can be attributed to the presence and role of Firmicutes [27].
3.2. Sharing of Microbiota
Out of a total of 118 genera, Clostridium showed abundance in most gut samples. The other dominant genera were Bacillus, Cyanobium, Aeromonas and Sphingomonas. Aeromonas was significantly abundant in the gut of wild varieties of Catla (78.2%) and common carp (61.2%). Sphingomonas and Bacillus have represented the gut of only cultured fish. The abundance of Cyanobium was significantly more in wild Rohu (54.8%) and cultured Rohu (50.3%). The cultured group shared only a small number of bacterial genera with the wild forms.
Out of the 64 genera reported in cultured Catla, 60 were not reported in the wild forms [Figure 2a]. Four genera were shared with the wild forms. The shared genera included Clostridium, Epulopiscium, Microcystis and Aeromonas.
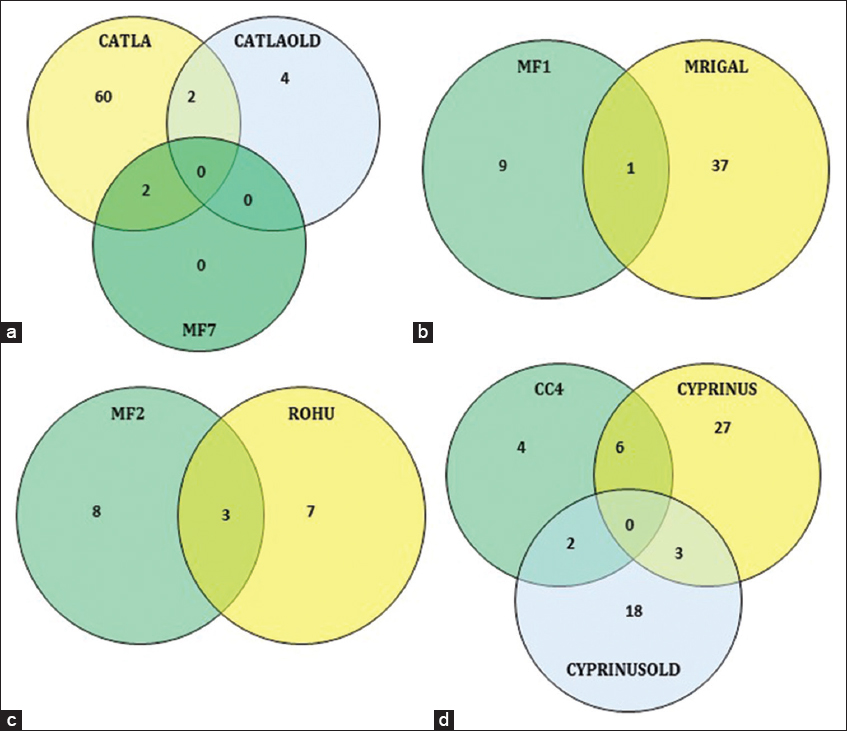 | Figure 2: Venn diagram comparing the shared and unique gut microbiome at the genus level. (a) Wild and cultured Catla; (b) wild and cultured Mrigal; (c) wild and cultured Rohu; (d) wild and cultured common carp. [Click here to view] |
Out of the 38 genera reported from cultured Mrigal, only 1 (Clostridium) was shared with wild Mrigal [Figure 2b].
In Rohu, 7 unique genera belonged to the fish from aquaculture farms while 8 different genera represented the wild type. 3 genera (Vibrio, Pirellula and Cyanobium) were common in both varieties [Figure 2c].
The cultured Common carp shared 11 genera with the wild forms. The shared genera included Aeromonas, Pasteurella, Pseudomonas, Corynebacterium, Mycobacterium, Brevibacterium, Kocuria, Rothia, Staphylococcus, Streptococcus, and Clostridium [Figure 2d].
Clostridium represented both wild and cultured forms. Clostridium can have an important role in digesting plant food by fermenting cellulose [28]. The abundance of Clostridium in the GI tract of herbivores and omnivores is more than in carnivores. To ferment cellulose, Clostridium produces digestive enzymes [27]. Clostridium butyricum has been found to inhibit the multiplication of pathogens by altering the gut microbiome in common carp [29].
The most dominant phylum was Cyanobacteria in both cultured and wild Rohu. The abundance of Cyanobacteria can be related to its contribution as the major food source as reported in earlier studies too [9]. A notable presence of Sphingomonas in the cultured carp cultured groups was observed in our study. Sphingomonas have a role in the degradation of xenobiotics as per earlier reports [30,31]. Pseudomonas, a Proteobacteria, was another dominant genus in both cultured and wild carp. The role of Pseudomonas in microplastic degradation [32], and antimicrobial activity [33] has already been reported. Bacillus was also the dominant genus in the cultured carp while wild groups did not show the presence of Bacillus. The representation of Bacillus in the gut of cultured forms might be offering many health-related advantages due to their capacity to produce cellulase [34], their ability to provide probiotic benefits and their role as fish pathogen inhibitors [35]. These results indicate that the wild and aquaculture habitats lead to variation in the gut microbiome of fish. This variation may be associated with the fish species in addition to their habitat.
3.3. Analysis of Alpha and Beta Diversities
The complexity of the species diversity was analyzed through Alpha diversity by means of Chao1, Shannon, Pielou’s evenness index and Faith’s Phylogenetic diversity. Alpha diversity data indicate significant differences (P-values: 0.019016474, 0.010515246, 0.010515246, and 0.055008834), respectively, among the examined four carp samples [Table 2]. The gastrointestinal microbial communities in carp from various habitats vary in diversity and richness, according to our findings.
Table 2: Analysis of α-diversity.
| ID | Habitat | Chaos 1 | Shannon entropy | Faith PD | Pielou’s evenness |
|---|---|---|---|---|---|
| CC4 | wild | 276 | 2.29 | 4.60 | 0.45 |
| MF1 | wild | 25 | 4.19 | 3.19 | 0.84 |
| MF2 | wild | 35 | 4.36 | 3.88 | 0.83 |
| MF7 | wild | 153 | 2.62 | 1.416 | 0.65 |
| CATLAOLD | wild | 99 | 2.24 | 2.47 | 0.50 |
| CYPRINUSOLD | wild | 31 | 4.42 | 8.867 | 0.69 |
| CATLA | culture | 38 | 6.74 | 17.26 | 0.86 |
| ROHU | culture | 16 | 5.64 | 16.12 | 0.88 |
| MRIGAL | culture | 238 | 6.34 | 35.16 | 0.84 |
| CYPRINUS | culture | 83 | 5.52 | 23.34 | 0.77 |
The Chao1 index reflects that the captive carp gut microbiome had slightly higher species richness than the wild carp studied. The Shannon index for cultured carps was higher than wild carps indicating that the species diversity is higher in fish that live in the captive environment. We observed the highest diversity in cultured Catla (Shannon index of 6.742358309) and Mrigal (Shannon index of 6.344573932) and the lowest in wild Catla (Shannon index of 2.244818315). Maximum phylogenetic diversity was observed in the case of cultured Mrigal (Faith’s Phylogenetic diversity of 35.16157526) and minimum in wild Catla (Faith’s Phylogenetic diversity of 41604475). This reflects that gut microbiome diversity in fish from aquaculture farms is higher than that in wild fish. This can be a result of the various feeds and habitat types. A similar observation has been made by Bereded et al. [10]. Ringo et al. [36] reviewed the impact of various dietary nutrient compositions and forms on the gut microbiome of fish. Some workers have observed higher microbial diversity in wild forms compared to cultured forms of fish such as Atlantic Salmon [37] and Malaysian Mahseer [38].
PCoA analysis showed significant differences in fish gut samples from wild and aquaculture settings. The PCoA plot [Figure 3] shows that the gut microbiome from the wild and the cultured samples are separated along PC1 representing 27.4% of the overall variation, except for one sample each of wild Catla (CATLAOLD) and Wild Cyprinus (CYPRINUSOLD). This can be an outcome of differences at the individual level. The gut microbiome of all the cultured and wild Rohu (ROHU and MF2, respectively) along with wild Catla (MF7) and Mrigal (MF1) were above PC2. The cultured fish gut samples of Catla (CATLA), Mrigal (MRIGAL), Cyprinus (CYPRINUS) and wild Catla (CATLAOLD), wild Cyprinus (CYPRINUSOLD) were below PC2, representing 22.1% of the overall variation. In conclusion, the two PCoA axes were able to account for more than 49% of the difference between the various communities. Clusters found in the UPGMA tree [Figure 4] of unweighted UniFrac distances were similar to the PCoA analysis. The cultured varieties of Catla, Mrigal and Common carp (CATLA, MRIGAL and CYPRINUS) clustered together with a wild variety of Common carp (CC4). The wild varieties of Catla, Mrigal and Rohu (MF7, MF1, and MF2, respectively) clustered together with the cultured variety of Rohu (ROHU). Unweighted unifrac PCoA [Figure 5] based on the number of features among the wild and cultured fishes shows that the gut microbiome of cultured forms clusters together while that of the wild forms cluster separately.
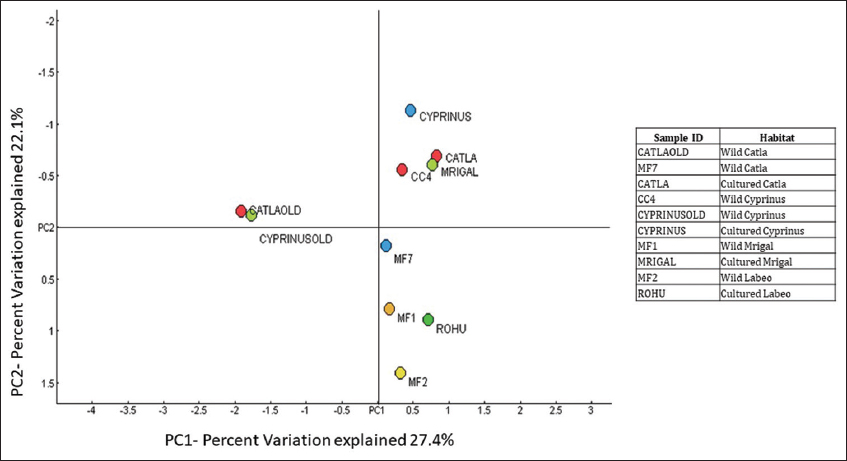 | Figure 3: PCoA of Taxonomy using Bray-Curtis PC1 (27.4%) versus PC2 (22.1%) at the genus level. [Click here to view] |
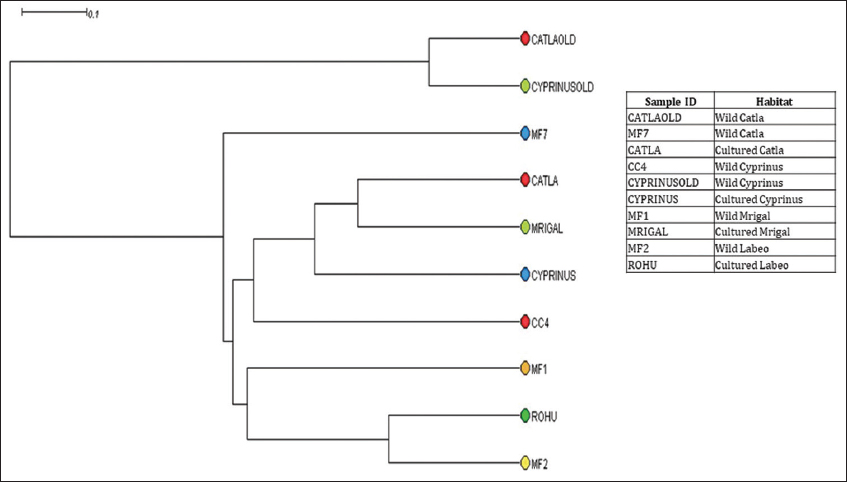 | Figure 4: Unweighted pair group method with arithmetic mean tree at genus level obtained after OTU table rarefied. [Click here to view] |
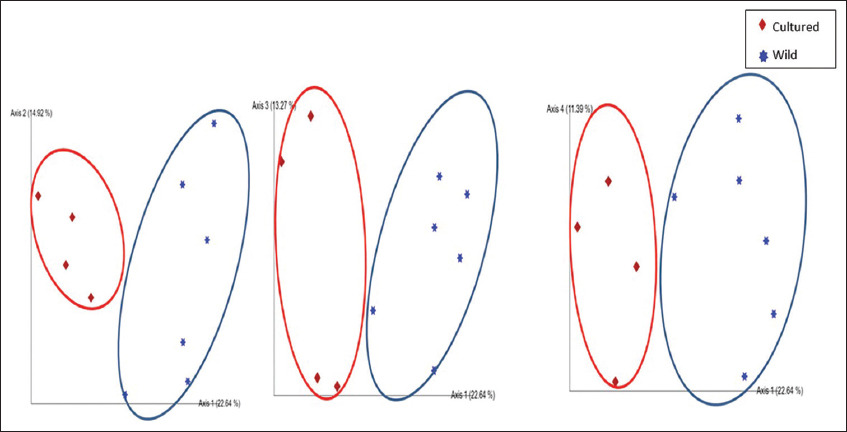 | Figure 5: Unweighted unifrac PCoA based on the number of features among the wild and cultured fishes. [Click here to view] |
From the analysis of the α-diversity indicators, it is clear that the gut microbiome of cultured fish showed more diversity than that of the group of wild carp studied. The β- diversity indicators (PCoA analysis) suggest that the gut microbiome of cultured fish resembles to a great extent. However, the gut microbiome of Rohu has striking dissimilarity with the wild and cultured forms of the other three groups.
4. CONCLUSION
This is the first report on the comparative account of the gut microbiome of Catla, Mrigal, Rohu and common carp together from wild and aquaculture settings. The gut microbiome analysis in the 4 fish groups from the aquaculture farms and wild was carried out using NGS. The wild and cultured groups exhibited a prominent dissimilarity in the composition and diversity of the gut microorganism. Our investigation reveals that the gut microbiome make-up varies in wild and cultured carp. The diversity of gut microbiome also varies considerably in the wild and cultured fish groups. The cultured carps exhibit more diversity of microbial communities. Furthermore, the occurrence of some genera such as Sphingomonas and Bacillus was comparatively high in cultured fish groups suggesting that they may offer survival benefits to cultured fish and may have colonized from the habitat. Since this is a pilot study, further investigations are needed on a larger sample size to derive concrete conclusions.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
6. FUNDING
There is no funding for the report.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
The authors confirm that the data supporting the findings of this study are available within the article and no supplementary data is required.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Beveridge MC, Thilsted SH, Phillips MJ, Metian M, Troell M, Hall SJ. Meeting the food and nutrition needs of the poor:The role of fish and the opportunities and challenges emerging from the rise of aquaculture. J Fish Biol 2013;83:1067-84. [CrossRef]
2. Fisheries and Aquaculture-cultured Aquatic Species-Labeo rohita. Available from:https://www.fao.org/fishery/en/culturedspecies/labeo_rohita/en [Last accessed on 2022 Oct 14].
3. Fisheries and Aquaculture-cultured Aquatic Species-Catla catla. Available from:https://www.fao.org/fishery/en/culturedspecies/catla_catla/en [Last accessed on 2022 Oct 14].
4. Fisheries and Aquaculture-cultured Aquatic Species-Cirrhinus mrigala.Available from:https://www.fao.org/fishery/en/culturedspecies/cirrhinus_mrigala/en [Last accessed on 2022 Oct 14].
5. Singh AK, Pathak AK, Lakra WS. Invasion of an exotic fish-common carp, Cyprinus CarpioL. (Actinopterygii:Cypriniformes:Cyprinidae) in the Ganga river, India and its impacts. Acta Ichthyol Piscat 2010;40:11-9. [CrossRef]
6. Yukgehnaish K, Kumar P, Sivachandran P, Marimuthu K, Arshad A, Paray BA, et al. Gut microbiota metagenomics in aquaculture:Factors influencing gut microbiome and its physiological role in fish. Rev Aquac 2020;12:1903-27. [CrossRef]
7. Kim PS, Shin NR, Lee JB, Kim MS, Whon TW, Hyun DW, et al. Host habitat is the major determinant of the gut microbiome of fish. Microbiome 2021;9:166. [CrossRef]
8. Meek RW, Vyas H, Piddock LJ. Nonmedical uses of antibiotics:Time to restrict their use?PLoS Biol 2015;13:e1002266. [CrossRef]
9. Ye L, Amberg J, Chapman D, Gaikowski M, Liu WT. Fish gut microbiota analysis differentiates physiology and behavior of invasive Asian carp and indigenous American fish. ISME J 2014;8:541-51. [CrossRef]
10. Bereded NK, Abebe GB, Fanta SW, Curto M, Waidbacher H, Meimberg H, et al. The impact of sampling season and catching site (wild and aquaculture) on gut microbiota composition and diversity of Nile Tilapia (Oreochromis niloticus). Biology (Basel) 2021;10:180. [CrossRef]
11. Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 2013;41:e1. [CrossRef]
12. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335-6. [CrossRef]
13. Edgar RC, Bateman A. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010;26:2460-1. [CrossRef]
14. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project:Improved data processing and web-based tools. Nucleic Acids Res 2013;41:D590-6. [CrossRef]
15. Huson DH, Beier S, Flade I, Górska A, El-Hadidi M, Mitra S, et al. MEGAN community edition-interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput Biol 2016;12:e1004957. [CrossRef]
16. Eichmiller JJ, Hamilton MJ, Staley C, Sadowsky MJ, Sorensen PW. Environment shapes the fecal microbiome of invasive carp species. Microbiome 2016;4:44. [CrossRef]
17. Zou S, Gong L, Khan TA, Pan L, Yan L, Li D, et al. Comparative analysis and gut bacterial community assemblages of grass carp and crucian carp in new lineages from the Dongting Lake area. Microbiologyopen 2020;9:e996. [CrossRef]
18. Parshukov AN, Kashinskaya EN, Simonov EP, Hlunov OV, Izvekova GI, Andree KB, et al. Variations of the intestinal gut microbiota of farmed rainbow trout, Oncorhynchus mykiss (walbaum), depending on the infection status of the fish. J Appl Microbiol 2019;127:379-95. [CrossRef]
19. Tyagi A, Singh B, Thammegowda NK, Singh NK. Shotgun metagenomics offers novel insights into taxonomic compositions, metabolic pathways and antibiotic resistance genes in fish gut microbiome. Arch Microbiol 2019;201:295-303. [CrossRef]
20. Maji UJ, Mohanty S, Mahapatra AS, Mondal HK, Samanta M, Maiti NK. Exploring the gut microbiota composition of Indian major carp, rohu (Labeo rohita), under diverse culture conditions. Genomics 2022;114:110354. [CrossRef]
21. Zeng A, Tan K, Gong P, Lei P, Guo Z, Wang S, et al. Correlation of microbiota in the gut of fish species and water. 3 Biotech 2020;10:472. [CrossRef]
22. Kersters K, De Vos P, Gillis M, Swings J, Vandamme P, Stackebrandt E. Introduction to the proteobacteria. In:The Prokaryotes. New York:Springer;2006. 3-37. [CrossRef]
23. Shu D, He Y, Yue H, Wang Q. Microbial structures and community functions of anaerobic sludge in six full-scale wastewater treatment plants as revealed by 454 high-throughput pyrosequencing. Bioresour Technol 2015;186:163-72. [CrossRef]
24. Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-Vitamin biosynthesis suggests co-operation among gut microbes. Front Genet 2015;6:148. [CrossRef]
25. Ottman N, Smidt H, de Vos WM, Belzer C. The function of our microbiota:Who is out there and what do they do?Front Cell Infect Microbiol 2012;2:104. [CrossRef]
26. Semova I, Carten JD, Stombaugh J, MacKey LC, Knight R, Farber SA, et al. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012;12:277-88. [CrossRef]
27. Li X, Yan Q, Xie S, Hu W, Yu Y, Hu Z. Gut microbiota contributes to the growth of fast-growing transgenic common carp (Cyprinus carpio L.). PLoS One 2013;8:e64577. [CrossRef]
28. Burgos FA, Ray CL, Arias CR. Bacterial diversity and community structure of the intestinal microbiome of Channel Catfish (Ictalurus punctatus) during ontogenesis. Syst Appl Microbiol 2018;41:494-505. [CrossRef]
29. Meng X, Wu S, Hu W, Zhu Z, Yang G, Zhang Y, et al. Clostridium butyricum improves immune responses and remodels the intestinal microbiota of common carp (Cyprinus carpio L.). Aquaculture 2021;530:735753. [CrossRef]
30. Ravintheran SK, Sivaprakasam S, Loke S, Lee SY, Manickam R, Yahya A, et al. Complete genome sequence of Sphingomonas paucimobilis AIMST S2, a xenobiotic-degrading bacterium. Sci Data 2019;6:280. [CrossRef]
31. Thelusmond JR, Strathmann TJ, Cupples AM. The identification of carbamazepine biodegrading phylotypes and phylotypes sensitive to carbamazepine exposure in two soil microbial communities. Sci Total Environ 2016;571:1241-52. [CrossRef]
32. Rajendran K, Rajendiran R, Ravichandran R, Velu RK. Investigation of microplastic accumulation in Rastrelliger kanagurta fish gut and microplastic degradation behaviour of existing gut bacteria Pseudomonas sp. Arch Microbiol 2022;204:626. [CrossRef]
33. Qi X, Xue M, Cui H, Yang K, Song K, Zha J, et al. Antimicrobial activity of Pseudomonas monteilii JK-1 isolated from fish gut and its major metabolite, 1-hydroxyphenazine, against Aeromonas hydrophila. Aquaculture 2020;526:735366. [CrossRef]
34. Ghosh K, Ray AK, Sen SK. Characterization of Bacilli isolated from the gut of Rohu, Labeo rohita, fingerlings and its significance in digestion. J Appl Aquac 2002;12:33-42. [CrossRef]
35. Santos RA, Oliva-Teles A, Pousão-Ferreira P, Jerusik R, Saavedra MJ, Enes P, et al. Isolation and characterization of fish-gut Bacillus spp. as source of natural antimicrobial compounds to fight aquaculture bacterial diseases. Mar Biotechnol (NY) 2021;23:276-93. [CrossRef]
36. RingøE, Zhou Z, Vecino JL, Wadsworth S, Romero J, Krogdahl A, et al. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story?Aquac Nutr 2016;22:219-82. [CrossRef]
37. Dehler CE, Secombes CJ, Martin SA. Environmental and physiological factors shape the gut microbiota of Atlantic salmon parr (Salmo salarL.). Aquaculture 2017;467:149-57. [CrossRef]
38. Tan CK, Natrah I, Suyub IB, Edward MJ, Kaman N, Samsudin AA. Comparative study of gut microbiota in wild and captive Malaysian Mahseer (Tor tambroides). Microbiologyopen 2019;8:e00734. [CrossRef]