1. INTRODUCTION
As in many other nations, tomato is one of the most significant, well-liked, and nutrient-dense vegetables in Indonesia. The relationship between plant growth and environmental factors has a significant impact on tomatoes’ capacity to yield fruit. Enhancing hydroponic farming methods is one way to combat the loss in the quality and quantity of tomato plant yields. The increased demand for food creates a need for novel plant variants, which further boosts crop output. In that instance, developing new, superior cultivars is thought of as one strategy for supplying people with food. Plant breeding is one method for developing improved cultivars. Genetic mutations are one illustration. Mutagens are frequently employed and useful in the research of genetics [1-3].
The capacity to enhance one or two exceptional traits of a plant without altering its genetic arrangement is the major benefit of mutant breeding. Mutations are especially useful in plant breeding since they reveal a lot about the numerous cell types in each plant as well as their different cell structures. Being the fundamental building blocks of plant growth and development, mutations serve as instruments for learning about natural life and cell function [4,5]. Crop productivity could be increased through the induction of genetic mutations [6,7].
Chemical and physical alterations can lead to genetic mutations. Alkylating substances, including ethyl methane sulfonate (EMS), are very efficient and manageable. Guanine (G) is frequently alkylated by EMS, resulting in O6-methylguanine, which couples with thymine (T) rather than cytosine (C), leading to C/G to T/A transitions. However, G/C to C/G or G/C to T/A transversions are also generated by 7-methylguanine hydrolysis, but less frequently [8]. EMS, diethyl sulfate, methyl methane sulphonate, hydroxylamine, sodium azide, and other compounds are frequently used to create chemical alterations. Ionizing radiation, beta radiation, neutron radiation, and gamma radiation can all cause physical mutations. The random amplified polymorphic DNA (RAPD) is deemed valuable and useful for detecting and documenting the diversity of tomato genotypes [9]. DNA is a practical tool for assessing genetic diversity [10-12].
Previous research has been successful in developing RAPD markers gene mapping using tomato plants as a source of material [13-15]. Using the RAPD approach, some tomato cultivars have been thoroughly investigated [16,17]. Potassium is a nutrient that plants absorb in high quantities, particularly in tomato plants. Fruits and vegetables are produced and of high quality as a result [18,19]. Furthermore, some research claims that potassium regulates the improvement in tomato output and quality [20-22].
Prior research demonstrates a relationship between water and the role of soluble sugar content in carbon allocation management the role of soluble sugar content in carbon allocation management. The addition of potassium will decrease starch and other carbon components during fruit ripening [23]. This study aimed to measure the genetic diversity of tomato plants following EMS and potassium treatments, as well as the effects of EMS concentration and potassium dose on tomato growth and yield.
2. MATERIALS AND METHODS
2.1. Materials
Some components used in this study were tomato seeds, EMS solution, potassium fertilizer, sodium thiosulfate, and RAPD – Polymerase chain reaction (PCR) kit.
2.2. Methods
The observations were conducted inside the greenhouse of the Faculty of Agriculture, the University of Jember, East Java, Indonesia. An EMS mutagen and potassium were utilized in the observation. Factorial randomized completely block design involving 4 replications was used in the experiment’s design.
The Duncan’s multiple range test was used to further examine the observational variables that showed a significant impact based on the findings of the test of variance (ANOVA) at a significance level of 5%. The concentration of EMS designated as E0, E1, and E2 correspondingly, at 0%, 0.3%, and 0.5% was the initial contributing component. Potassium (K) was administered in doses of 0 g, 2.5 g, and 5 g, which were designated as K0, K1, and K2, respectively.
2.2.1. Seed preparation
The seeds were steeped in sterile water for 8 h inside the nylon mesh. The seeds were then soaked for 24 h in a 50 mL of EMS solution (Sigma Chemical Company, USA) at 0.3% and 0.5% rates while being gently stirred. The EMS solution was then withdrawn, and the seeds were twice washed with 3% sodium thiosulfate buffer while being gently shaken for 15 min, and then washed for 5 min with distilled water.
After removing the EMS mixture, the seeds were then twice rinsed with 3% sodium thiosulfate buffer while being gently shaken for 15 min, followed by a 5-min wash with distilled water.
2.2.2. Plant cultivation
When the treated seeds had sprouted in nursery media for 21 days, the plants were moved into a pot. In the greenhouse, the plants were raised. The tomatoes were harvested for the organic substance analysis when they reached the color-breaking phase or the end of the fruit ripening phase.
2.2.3. Fruit content determination
The Bradford (1976) method was modified somewhat [24] to ascertain the samples’ whole dissolved protein concentration. While Vitamin C was measured using ultraviolet-spectrophotometric analysis, reducing sugars were measured using the Nelson-Somogyi method.
2.2.4. RAPD
Seven primers were used for the RAPD - PCR: OPC-08-TGG ACC GGT G; OPC- 11-AAA GCT GCG G; OPC-20-ACT TCG CCA C; and OPE-07-AGA TGC AGC C. The PCR reaction was performed in accordance with the instructions in the kit’s manual. The examination results of the RAPD data are shown in Table 1. The electrophoresis’s results were then subjected to gel analysis. For each DNA band that was not present, the RAPD analysis gel received a score of 0/1. The findings demonstrated the utility of the RAPD technique for the creation of genetic maps and the detection of polymorphisms in tomato plant genotypes. Calculations were utilized to establish the total number of bands, the number of polymorphic bands, and the polymorphic information content (PIC) [25,26].
Table 1: Results of analysis of variance on parameters of Vitamin C levels, reducing sugars, total protein, and Brix content.
| Parameters | Treatment | F-test | Notation |
|---|---|---|---|
| Vitamin C | EMS | 376.59 | ** |
| Potassium | 490.55 | ** | |
| EMS*Potassium | 594.56 | ** | |
| Sugar reducing | EMS | 318.98 | ** |
| Potassium | 20.01 | ** | |
| EMS*Potassium | 571.51 | ** | |
| Total protein | EMS | 151025.43 | ** |
| Potassium | 87043.24 | ** | |
| EMS*Potassium | 221252.62 | ** | |
| Brix content | EMS | 2.47 | ns |
| Potassium | 2.20 | ns | |
| EMS*Potassium | - | - |
** Mean that significantly, ns mean that not significantly different at 5% or 1% level based on analysis of variance test
3. RESULTS AND DISCUSSION
3.1. The Effect of Mutations using EMS and Potassium Fertilizer Application
Based on the findings obtained from the analysis of variance, parameters of vitamin C levels were significantly affected by the EMS and potassium treatments, reducing sugars, and total dissolved protein at a significance level of 1%. Each treatment using potassium and EMS showed a different significant effect according to the findings of analysis of variance. EMS and potassium treatment and the mutual interaction showed a significant effect on the parameters of vitamin C, reducing sugars, and total dissolved protein. The EMS and potassium treatments alone showed no significant effect on the Brix value parameter. The F value of the results of the analysis of variance for each treatment of the observation parameters is displayed in Table 1.
Treatments using EMS and potassium [Figure 1] showed different effects on the vitamin C concentration of the fruit. The best treatment result was obtained using 0% EMS and 2.5 grams of potassium with a vitamin C content of 0.94 mg/g. The next best vitamin C levels in fruit were then shown to be the result of treatment using 0.3% EMS and 0 grams of potassium with a yield of 0.91 mg/g. The next best vitamin C content was from the treatment using 0.5% EMS and 2.5 grams of Potassium with a yield of 0.78 mg/g. The lowest vitamin C content was shown in the treatment using 0.3% EMS and 5 g of potassium which was 0.52 mg/g. Potassium is involved in the metabolism between the formation of carbohydrates and ascorbic acid [27]. The role of potassium as a precursor for various enzymes is thought to increase the formation of ascorbic acid through D-glucose and D-galactose.
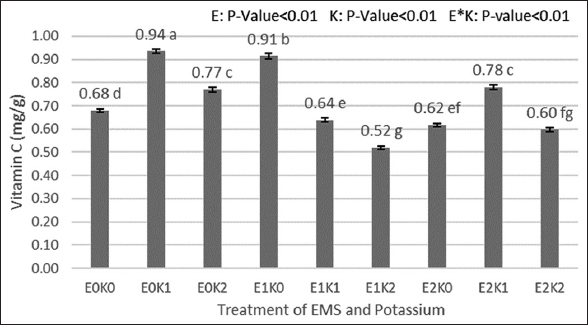 | Figure 1: Effects of ethyl methane sulfonate and Kalium on vitamin C content. [Click here to view] |
Vitamin C level was measured to determine the effect of treatments utilizing EMS and potassium on fruit quality. The fruit with the highest vitamin C content was the result of the treatment using 0% EMS combined with 2.5 g of potassium. Then, the fruit with the lowest amount of vitamin C was from treatment using 0.3% EMS combined with 5 g of potassium.
Treatment that produced the maximum reducing sugar was performed using 0.3% of EMS concentration without potassium, while the treatment producing the least reducing sugar was performed using 0.5% of EMS, excluding the K treatment. In his experiment [28]. Vitamin C content increased with an increase in K concentration, however, this did not show a statistically significant difference. The amylase enzyme, in general, breaks down carbs into sugar to give tomatoes their characteristically sweet flavor [29,30].
Bulawa et al. [31] stated that in Brassica juncea cultivars, potassium administration enhanced total chlorophyll efficiency, which in turn increased photosynthetic efficiency, plant growth, total phenols, flavonoids, and Vitamin C levels. Meanwhile [32] state that total soluble solids, vitamin C content, total sugar, and fruit surface color of pepper fruit were all improved by potassium fertilizer.
The usage of potassium fertilizer and the amount of vitamin C in plants are positively correlated, according to several research [33]. Photosynthesis depends heavily on potassium [34] because ascorbate and ascorbate peroxidases work together to scavenge the abundant hydrogen peroxide created in the time of photosynthesis in conditions with high irradiation and a clear relationship involving photosynthesis and vitamin is found [35].
Glucose, sucrose, and fructose are the sugars that are created during the breakdown process. The sweetness of any plant product is governed by this sort of sugar. Fruit’s sweetness was assumed to be related to the amount of sugar present, especially decreasing sugar (glucose and fructose) .
The highest reducing sugar [Figure 2] was obtained in the treatment using 0.3% EMS and 0 g of potassium which was 2.47 mg/g. The highest reducing sugar yield was then shown in the treatment using 0% EMS and 0.25 g of potassium with a yield of 1.59 mg/g. The next best treatment used 0% EMS and 0.5 g of potassium and 0.5% EMS and 0.25 g of potassium with a result of 1.52 mg/g and 1.44 mg/g. The treatment that produced the least reducing sugars was 0.5% of EMS and 0 g of potassium with a result of 0.94 mg/g.
 | Figure 2: Effects of ethyl methane sulphonate and Kalium on reducing sugar content. [Click here to view] |
Potassium is the greatest prevalent inorganic cation and is necessary for fostering strong growth of crop (K) [36]. Many crucial enzymes, including those involved in protein synthesis, sugar transport, N and C metabolism, and photosynthesis, are activated by K. This element is crucial for yield growth and quality improvement [37,38]. Because the amount of sugar in plants is positively correlated with the sufficiency of the potassium element, the amount of reducing sugar will rise if potassium fertilizer is applied more frequently [39]. Fruit sugar is imported from the source to the sink, and as potassium affects how well sugar is transported, potassium will also alter fruit sugar [40,41].
The combined treatment with 0.3% EMS, however, produced the highest sugar content in the treatment without the addition of potassium elements. Potassium, a component of the basic fertilizers applied at planting time, has a considerable impact on sugar yield. It was anticipated that this would likewise have the EMS treatment’s impact on the plant’s metabolism.
Our result showed that the treatment with 0.3% EMS and 0 grams of potassium produced the highest production of total dissolved protein, with a yield of 22.33 mg/g. The highest total dissolved protein yield was further shown in the treatment using 0.5% EMS and 5 g of potassium with a yield of 19.66 mg/g. The treatment using 0.5% EMS and 0.25 g of potassium showed the lowest total dissolved protein yield of 7.31 mg/g. In light of the findings of the [42] experiment, demonstrating that EMS treatment increased the protein and oil content of soybean seeds. Meanwhile, according to [43] the 0.3% EMS treatment on mulberry plants showed the highest protein content compared to the 0.1% EMS treatment and controls.
When measuring the dissolved protein in tomato leaf extract (using Standard BSA), the combined effects of mutagen and potassium were highly significant [Figure 3]. The total dissolved protein in tomato leaf samples is also considerably influenced by single-factor EMS and potassium. The treatment with the greatest overall soluble protein content used 0.3% of EMS and no potassium. Meanwhile, the treatment utilizing 0.5% of EMS and 2.5g of potassium had the lowest total dissolved protein content. The amount of seed protein and oil content can be increased through treatment utilizing EMS in conjunction with gamma-ray irradiation [27,42]. According to [44] Potassium (K) and nitrogen (N), which are commonly used as fertilizers in agricultural production, play a critical role in nitrogen metabolism. Potassium accelerated the rate of amino acid translocation into grains as well as the conversion of amino acids into grain proteins (albumin, globulin, prolamin, and glutelin). The accumulation of free amino acids in the grain treated with K2 indicates that the effects of K on amino acid translocation were more important than those on amino acid production. It can be concluded that amino acid translocation into the grain, not K+’s influence on peptide bond formation, leads to an indirect effect of K+ on grain protein production [45].
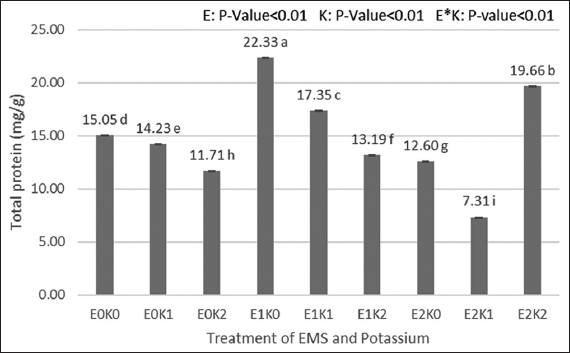 | Figure 3: Effects of ethyl methane sulfonate and Kalium on total protein content. [Click here to view] |
Mutations due to EMS cause genetic variation due to changes in the genome structure which can be in the form of differences in the number and size of alleles in mutant plants [46], can also induce random points or create new stop codons in genes [47]. Mutations due to EMS cause genetic variation due to changes in the genome structure which can be in the form of differences in the number and size of alleles in mutant plants [46]; in addition, it can also induce random points or create new stop codons within the genes [47]. Mutations due to EMS cause genetic variation due to changes in the genome structure which can be in the form of differences in the number and size of alleles in mutant plants [46], can also induce random points or create new stop codons in genes [47]. Mutations due to EMS cause genetic variation due to changes in the genome structure which can be in the form of differences in the number and size of alleles in mutant plants [46]; in addition, it can also induce random points or create new stop codons within the genes [47].
The vital nutrient potassium has an impact on the physiological and biochemical mechanisms that significantly control the growth and metabolism of crops. Potassium is necessary for a number of biochemical and physiological processes that are involved in the development of plants and their growth. Protein synthesis, glucose metabolism, and enzyme activation are all influenced by potassium [48,49]. Fascinating findings show that adding potassium to the treatment has no impact on protein content. The protein concentration was maximum even without the addition of potassium components. It will be fascinating to see if the EMS therapy affects how element K is metabolized in the safe cells.
The recent study’s findings also showed that Brix value was also measured to determine EMS combined with potassium treatment to tomatoes [Figure 4]. The result showed that 0.3% EMS combined with 5 g of potassium had the highest Brix value (5.0oBrix) and the treatment using 0.5% EMS combined without potassium had the lowest Brix value (3.9oBrix). The maximum Brix value was obtained with EMS treatment at doses of 0.3% without the addition of potassium fertilizer (4.9oBrix). The addition of potassium up to a dose of 5 g in plants mutated with 0.3% and 0.5% EMS tended to increase the brix value of tomatoes. The addition of potassium had a greater effect on the variable value of brix or total dissolved solids (TSS) and gave a significant response [50]. The increase in sugar proved the significance of potassium on the process of biosynthesis and transfer of sugars throughout the plant body [51].
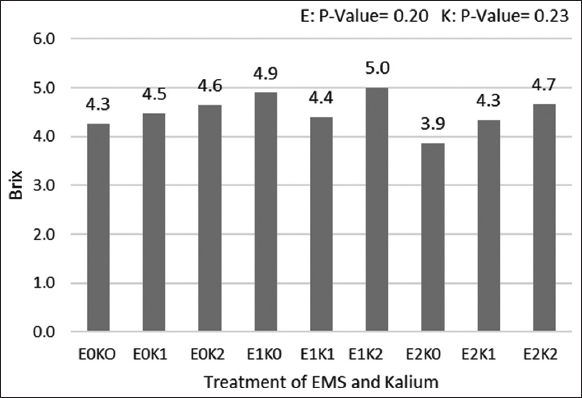 | Figure 4: Effects of ethyl methane sulfonate and Kalium on Brix content of tomato. [Click here to view] |
Likewise [22] identified that sugars make up the majority of the tomato’s total soluble solids, which are what give the fruit its flavor and other qualities. The outcome shown that potassium levels had a substantial impact on oBrix.
According to Wuzhong [52], increasing the application of the K fertilizer may raise the concentration of sugars in tomatoes which could increase the TSS content of tomatoes due to increased imports and sugar buildup [53] [54]. Stated that K treatment had no appreciable impact on tomato Brixo, in contrast to current data.
Meanwhile, Brix 9-2-5, the gene that codes for the relevant amino acid polymorphisms of cell wall invertase, is known to regulate the fruit sugar concentration in tomatoes (LIN5). This has a significant impact on fruit sink strength and alters the kinetics of sugar concentration [55].
It can be concluded that EMS treatment changed the capacity of tomato plants to respond to environmental cues since soluble solids in tomatoes were more influenced by environmental factors [56,57]. It is reasonable to conclude that tomato plant’s capacity to respond to their surroundings was impacted by EMS treatment. This is consistent with discoveries of similar correlations in organic molecules.
3.2. Identification of Plant Genotype Changes Resulting from Mutations of EMS
A mutagen that modifies the nitrogen base is EMS. At several spots on the nitrogen base, EMS inserts acyl ethyl groups (-CH2CH3) that alter the base’s structure and ultimately lead to the incorrect pair [Figure 5]. The molecular formula for the EMS substance is C3H8SO3. The nucleotide chain may become methylated because of EMS mutagens. Guanine (G) and cytosine (C), two DNA molecular bases, are mismatched during replication as a result of the methylation process [58,59].
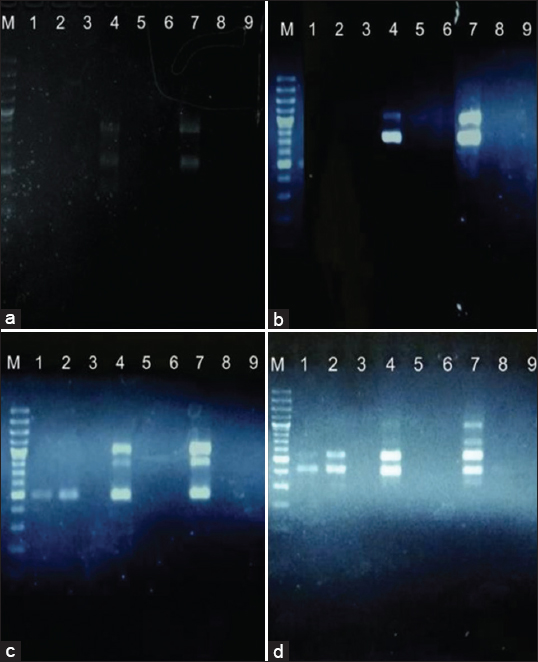 | Figure 5: DNA visualization of the ethyl methane sulphonate treatment in tomato plants by utilizing random amplified polymorphic DNA Markers of OPC-08 (a), OPC-11 (b), OPC-20 (c), and OPE-07 (d). (1; E0K0, 2; E0K1, 3; E0K2, 4; E1K0, 5; E1K1, 6; E1K2, 7; E2K0, 8; E2K1, 9; E2K2, and M; Marker). [Click here to view] |
Genetic alterations may result from using EMS on tomatoes. Using RAPD Polymorphic markers (Random Amplified DNA), kinship analysis can be performed to determine DNA alterations at various EMS concentrations. This study used four primers and RAPD markers to find genotype alterations in tomato plants.
Utilizing RAPD marker-based OPB-08 and OPC-11 primers, the findings of DNA visualization after administering EMS to tomato plants revealed a total of 2 polymorphic DNA bands. Visually, only two of the nine samples, for example, sample 4 (E1K0 treatment) and sample 7—were amplified (E2K0 treatment) . It can be inferred that there was no significant change in the genotypes of the two treatments because the amplification of the DNA produced by the two treatments using OPB-08 and OPC-11 primers produced the same findings at the same size.
A total of three DNA bands, including one polymorphic band and two monomorphic bands, were obtained after using OPC-primers 20. Four of the nine samples (E2K0 treatment)—samples 1 (E0K0 treatment), 2 (E0K1 treatment), 4, and 7 (E1K0 treatment)—were amplified. The three samples that showed all three DNA bands, namely samples 1 (E0K0 treatment), 2 (E0K1 treatment), and 4 (E1K0 treatment) were comparable to sample 2 (E0K1 treatment), which showed each DNA band enhanced.
Five DNA bands were found using the OPE-08 primer, 3 of which were polymorphic and 2 of which were monomorphic. Out of the nine samples (E1K0 and E2K0 treated) were visual samples 1 (E0K0 treated), 2 (E0K1 treated), 4, and 7. There was no difference between the three samples in the OPE-primers 08 experiment since sample 1 (E0K0 treatment), sample 2 (E0K1 treatment), and sample 4 (E1K0 treatment) all shared traits that suggested that each of the two DNA bands had the same size. Based on the detection of DNA from four primers, only three of the nine samples, including samples 1, 2, and 7, were amplified.
Non-emergence in samples 3 (E0K2 treatment), 5, 6, 8, and 9 (E1K2 treatment) was caused by several variables, including non-isolated DNA templates. High lycopene molecules may have an impact on tomato plants’ ability to isolate their DNA. The incompatibility of the site with the primer was another factor.
Table 2 displays the total number of DNA bands and the percentage of polymorphisms produced by the utilized primers. Based on amplified DNA bands, they can be divided into monomorphic and polymorphic patterns. Monomorphic DNA band patterns are those that possess the same number and size of DNA bands, while polymorphic band patterns are the opposite. With a median of 3 bands for each primer, a total of 12 DNA bands were amplified. 73.3% of the polymorphism was produced by the 4 primers that were utilized. A significant percentage of polymorphisms (> 50%) can be used to explain the genetic differences in the observed group [60,61].
Table 2: The percentage of polymorphisms and the quantity of DNA bands and in tomato plants treated using EMS.
| No. | Primary name | Sequence (5’-3 ’) | DNA | Band polymorphic | Percentage polymorphism (%) |
|---|---|---|---|---|---|
| 1. | OPC-08 | TGG ACC GGT G | 2 | 2 | 100 |
| 2. | OPC-11 | AAA GCT GCG G | 2 | 2 | 100 |
| 3. | OPC-20 | ACT TCG CCA C | 3 | 1 | 33.3 |
| 4. | OPE-07 | AGA TGC AGC C | 5 | 3 | 60 |
| Total | 12 | 8 | 293.3 | ||
| Average | 3 | 2 | 73.3 |
EMS: Ethyl methane sulfonate
We hypothesize that the varying percentage of G/C to A/T conversion brought about by the EMS treatment may be connected to the genetic makeup and/or genomic features of these plants. According to research (2003), 192 target genes in Arabidopsis plants had a G/C to A/T transition responsible for more than 99% of the EMS-induced variations.
It is clear from the overall DNA visualization data [Figure 5] that the genotype structure of tomato plants is influenced by the administration of EMS at various concentrations. Ethylguanin, an aberrant base created by methylation caused by EMS mutagens, is the result. Ethylguanine will be recognized by the DNA polymerase enzyme as thymine when it is coupled with adenine. A plant’s morphology will be impacted by the application of EMS to induce mutations that resulted in Genetic changes. Plant genetic diversity can be produced through induction using a variety of EMS concentrations [59,62,63].
Cluster analysis was used to determine the various genotypes of the EMS treatment. A dendrogram generated by cluster analysis can be used to compare treatments and identify genetic distances. Two clusters were created using the dendrogram mentioned above. Given that E0K0 and E0K1 (treatments without EMS and at various potassium doses) occupied Cluster I, it may be deduced that genotype modifications in tomato plants were unaffected by the application of various potassium dosages [Figure 6].
 | Figure 6: Dendrogram genotype of ethyl methane sulphonate treatment in tomato plants using random amplified polymorphic DNA markers using UPGMA Jaccard’s Index. [Click here to view] |
In comparing E0K0 and E0K1, there was no genotype difference and a genetic divergence of 1.000 was found [Table 3]. E1K0 and E2K0 were the only individuals in Cluster II and their genetic distance was 0.750 [Table 3]. The difference in EMS administration between the two treatments revealed genetic variations in each. The E2K0 treatment (0.5% of EMS dose and 25 g of potassium fertilizer/plant) induced more polymorphism than the E1K0 treatment (0.1% EMS dose and 25 g of potassium fertilizer/plant).
Table 3: Genetic distance EMS and potassium fertilizer treatment in tomato plants.
| P | E0K0 | E0K1 | E1K0 | E2K0 |
|---|---|---|---|---|
| E0K0 | 1.000 | |||
| E0K1 | 1.000 | 1.000 | ||
| E1K0 | 0.500 | 0.500 | 1.000 | |
| E2K0 | 0.250 | 0.250 | 0.750 | 1.000 |
EMS: Ethyl methane sulfonate
Based on the observed DNA banding pattern, Table 3 compares the genetic distance between treatments. When the tile distance is more than 70% or 0.700, there is little to no genetic variation. The distance between the 4 samples is described in this table. To assess changes in genotypes brought on by EMS and potassium fertilizers, more study is required for the other 5 samples [64,65].
The genetic differences between tomato plants E1K0 and E2K0, which have a genetic distance of 0.750, are brought by the application of various doses of EMS. The genetic changes in tomato plants are unaffected by potassium administration at various doses. In 4 DNA samples amplified from 4 primers, kinship analysis creates 2 groups. The 12 DNA bands produced by the employed primers had 73.3% polymorphisms. The highest amount of sweetness is produced by mutation induction at a concentration of 0.3%, while the most Vitamin C is produced at a concentration of 0.5%.
4. CONCLUSIONS
Tomato crops, notably E1K0 and E2K0 with a genetic divergence of 0.750, experience genetic changes in response to various EMS doses. The genetic alterations in tomato plants are unaffected by potassium administration at various levels. In 4 DNA samples amplified from 4 primers, kinship analysis forms 2 groups. The 12 DNA bands produced by the employed primers had 73.3% polymorphisms. At a concentration of 0.3%, mutation induction yields the most sweetness, whereas, at a concentration of 0.5, it yields the most Vitamin C.
5. ACKNOWLEDGMENTS
The authors are thankful to the LP2M University of Jember for the support in this research through the KERIS Horticulture program.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All data generated and analyzed are included within this research.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Chaudhary J, Alisha A, Bhatt V, Chandanshive S, Kumar N, Mir Z, et al. Mutation breeding in tomato:Advances, applicability and challenges. Plants (Basel) 2019;8:128. [CrossRef]
2. Adamu AK, Aliyu H. Morphogical effects of sodium azide on tomato (Lycopersicon esculentum Mill). Sci World J 2007;2:9-12. [CrossRef]
3. Kozgar MI, Khan S, Wani MR. Variability and correlations studies for total iron and manganese contents of chickpea (Cicer arietinum L.) high yielding mutants. Am J Food Technol 2012;7:437-44. [CrossRef]
4. Akhtar A, Fuchs E, Mitchison T, Shaw RJ, St Johnston D, Strasser A, et al. A decade of molecular cell biology:Achievements and challenges. Nat Rev Mol Cell Biol 2011;12:669-74. [CrossRef]
5. Reichert NA. History of plant genetic mutations ±human influences. In Vitro Cell Dev Biol Plant 2021;57:554-64. [CrossRef]
6. Sikora P, Chawade A, Larsson M, Olsson J, Olsson O. Mutagenesis as a tool in plant genetics, functional genomics, and breeding. Int J Plant Genomics 2011;2011:314829. [CrossRef]
7. Kodym A, Afza R. Physical and chemical mutagenesis. In:Plant Functional Genomics. Berlin, Germany:Springer:2003. 189-203. [CrossRef]
8. Krieg DR. Ethyl methanesulfonate-induced reversion of bacteriophage T4rII mutants. Genetics 1963;48:561-80. [CrossRef]
9. Salunke DS, Jadhav AS, Pawar BD, Kale AA, Chimote VP. Diversity analysis of tomato genotypes using RAPD markers and high-performance liquid chromatography in relation to β- carotene content. Vegetos 2012;25:95-101.
10. Park EJ, Jekni?Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata N, et al. Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J 2004;40:474-87. [CrossRef]
11. Semagn K, Bjørnstad A, Ndjiondjop MN. An overview of molecular marker methods for plants. Afr J Biotechnol 2006;5:2540-68.
12. Mondini L, Noorani A, Pagnotta MA. Assessing plant genetic diversity by molecular tools. Diversity 2009;1:19-35. [CrossRef]
13. Foolad MR. Genome mapping and molecular breeding of tomato. Int J Plant Genomics 2007;2007:64358. [CrossRef]
14. Klein-Lankhorst RM, Vermunt A, Weide R, Liharska T, Zabel P. Isolation of molecular markers for tomato (L. esculentum) using random amplified polymorphic DNA (RAPD). Theor Appl Genet 1991;83:108-14. [CrossRef]
15. Ranc N, Muños S, Xu J, Le Paslier MC, Chauveau A, Bounon R, et al. Genome-wide association mapping in tomato (Solanum lycopersicum) is possible using genome admixture of Solanum lycopersicum var. Cerasiforme. G3 (Bethesda) 2012;2:853-64. [CrossRef]
16. Noli E, Cont S, Maccaferri M, Sanguineti MC. Molecular characterization of tomato cultivars. Seed Sci Technol 1999;27:1-10.
17. Singh N, Singh M, Kumar S, Kumar R, Singh V, Prasanna HC, et al. RAPD markers for hybrid seed purity testing in tomato (Solanum lycopersicum L.). Cur Sci 2007;93:462-3.
18. Mengel K, Kirkby EA. Principles of Plant Nutrition. 4th ed. Switzerland, Worblaufen-Bern:International Potash Institute;1987.
19. Sultana R, Dilruba S, Parvin N, Islam AB. Effect of potassium on growth and yield of tomato (Lycopersicon esculentum Mill.) Ecofriendly Agric J 2015;8:77-80.
20. Fontes PC, Sampaio RA, Mantovani EC. Tomato yield and potassium concentrations in soil and in plant petioles as affected by potassium fertirrigation. Pesqui Agropecuária Bras 2000;35:575-80. [CrossRef]
21. Çolpan E, Zengin M, Özbahçe A. The effects of potassium on the yield and fruit quality components of stick tomato. Hortic Environ Biotechnol 2013;54:20-8. [CrossRef]
22. Woldemariam SH, Lal S, Zelelew DZ, Solomon MT. Effect of potassium levels on productivity and fruit quality of tomato (Lycopersicon esculentum L.). J Agric Stud 2018;6:102-15. [CrossRef]
23. Luo A, Kang S, Chen J. SUGAR model-assisted analysis of carbon allocation and transformation in tomato fruit under different water along with potassium conditions. Front Plant Sci 2020;11:712. [CrossRef]
24. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding Anal Biochem 1976;72:248-54. [CrossRef]
25. Anderson JA, Churchill GA, Autrique JE, Tanksley SD, Sorrells ME. Optimizing parental selection for genetic linkage maps. Genome 1993;36:181-6. [CrossRef]
26. Foolad MR, Jones RA, Rodriguez RL. RAPD markers for constructing intraspecific tomato genetic maps. Plant Cell Rep 1993;12:293-7. [CrossRef]
27. Espina MJ, Ahmed CM, Bernardini A, Adeleke E, Yadegari Z, Arelli P, et al. Development and phenotypic screening of an ethyl methane sulfonate mutant population in soybean. Front Plant Sci 2018;9:394. [CrossRef]
28. Shabani SE, Tabatabaei SJ. The effect of potassium concentration in nutrient solution on lycopene, Vitamin C and qualitative characteristics of cherry tomato in saline conditions. J Crop Prod Process 2013;3:133-43.
29. Alam MJ, Rahman MH, Mamun MA, Ahmad I, Islam K. Enzyme activities in relation to sugar accumulation in tomato. Proc Pak Acad Sci 2006;43:241-8.
30. Zhang Y, Ma C, Delohery T, Nasipak B, Foat BC, Bounoutas A, et al. Identification of genes expressed in C. elegans touch receptor neurons. Nature 2002;418:331-5. [CrossRef]
31. Bulawa B, Sogoni A, Jimoh MO, Laubscher CP. Potassium application enhanced plant growth, mineral composition, proximate and phytochemical content in Trachyandra divaricata Kunth (Sandkool). Plants 2022;11:3183. [CrossRef]
32. Botella MA, Arévalo L, Mestre TC, Rubio F, García-Sánchez F, Rivero RM, et al. Potassium fertilization enhances pepper fruit quality. J Plant Nutr 2017;40:145-55. [CrossRef]
33. Shehata SA, El-Mogy MM, Mohamed HF. Postharvest quality and nutrient contents of long sweet pepper enhanced by supplementary potassium foliar application. Int J Veg Sci 2019;25:196-209. [CrossRef]
34. Zahoor R, Dong HR, Abid M, Zhao WQ, Wang YH, Zhou ZS. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ Exp Bot 2017;137:73-83. [CrossRef]
35. Fenech M, Amaya I, Valpuesta V, Botella MA. Vitamin C content in fruits:Biosynthesis and regulation. Front Plant Sci 2006;9:2006. [CrossRef]
36. White PJ, Karley AJ. Potassium Cell Biology of Metals and Nutrients. Berlin:Springer;2010. 199-224. [CrossRef]
37. Marschner H. Marschner's Mineral Nutrition of Higher Plants. Cambridge, MA:Academic Press;2012.
38. Oosterhuis DM, Loka DA, Kawakami EM, Pettigrew WT. The physiology of potassium in crop production. Adv Agron 2014;126:203-3. [CrossRef]
39. Zushi K, Matsuzoe N. Effect of soil water deficit Vitamin C, sugar, organic acid, amino acid and carotene contents of large-fruited tomatoes. J Japan Soc Hortic Sci 1998;67:927-33. [CrossRef]
40. Caretto S, Parente A, Serio F, Santamaria P. Influence of potassium and genotype on Vitamin E content and reducing sugar of tomato fruits. Hortscience 2008;43:2048-51. [CrossRef]
41. Almeselmani M, Pant RC, Singh B. Potassium level and physiological response and fruit quality in hydroponically grown tomato. Int J Veg Sci 2009;16:85-99. [CrossRef]
42. Pavadai P, Girija M, Dhanavel D. Effect of gamma rays, EMS, DES and COH on protein and oil content in soybean. J Ecobiotechnol 2010;2:47-50.
43. Kumar HV, Muralidhar TS, Acharya S, Das MJ. EMS induced morphometric biomass and phytochemical variations in Morus species (genotype RFS135). Am J Exp Agric 2013;3:43-55. [CrossRef]
44. Xu X, Du X, Wang F, Sha J, Chen Q, Tian G, et al. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front Plant Sci 2020;11:904. [CrossRef]
45. Mengel K, Secer M, Koch K. Potassium effect on protein formation and amino acid turnover in developing wheat grain. Agron J 1981;73:8. [CrossRef]
46. Dwinianti EF, Mastuti R, Arumingtyas EL. Genetic variation analysis of EMS-induced chili pepper (Capsicum frutescens L.) mutants using SSR markers. J Trop Life Sci 2019;9:223-8. [CrossRef]
47. Siddique MI, Back S, Lee JH, Jo J, Jang S, Han K, et al. Development and characterization of an ethyl methane sulfonate (EMS) induced mutant population in Capsicum annuum L. Plant (Basel) 2020;9:396. [CrossRef]
48. Wang M, Zheng Q, Shen Q, Guo S. The critical role of potassium in plant stress response. Int J Mol Sci 2013;14:7370-90. [CrossRef]
49. Sardans J, Peñuelas J. Potassium control of plant functions:Ecological and agricultural implications. Plants (Basel) 2021;10:419. [CrossRef]
50. Moreira LC, Silva LD, Nascimento BM, Silva AJ, Teixeira AD, de Oliveira MR. Agronomic performance and fruits quality of yellow melon fertilized with doses of nitrogen and potassium. Rev Catinga 2022;35:320-30. [CrossRef]
51. Javaria S, Khan MQ, Bakhsh I. Effect of potassium on chemical and sensory attributes of tomato fruits. J Anim Plant Sci 2012;22:1081-5.
52. Wuzhong N. Yield and quality of fruits of solanaceous crops as affected by potassium fertilization. Better Crops Int 2002;16:6-8.
53. Balibrea ME, Martínez-Andújar C, Cuartero J, Bolarín MC, Pérez-Alfocea F. The high fruit soluble sugar content in wild Lycopersicon species and their hybrids with cultivars depends on sucrose import during ripening rather than on sucrose metabolism. Funct Plant Biol 2006;33:279-88. [CrossRef]
54. Al-Moshileh AM, Errebhi MA, Obiadalla-Ali HA. Effect of potassium fertilization on tomato and cucumber plants under greenhouse conditions. Biosci Res 2017;14:68-74.
55. Fridman E, Carrari F, Liu YS, Fernie AR, Zamir D. Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science 2004;305:1786-9. [CrossRef]
56. Helyes L, Pek Z. Tomato fruit quality and content depend on stage of maturity. Hortscience 2006;41:1400-1. [CrossRef]
57. Klap C, Yeshayahou E, Bolger AM, Arazi1 T, Gupta SK, Shabtai S, et al. Tomato facultative parthenocarpy results from SLAGAMOUS-LIKE 6 loss of function. Plant Biotechnol J 2017;15:634-47. [CrossRef]
58. Angers B, Castonguay E, Massicotte R. Environmentally induced phenotypes and DNA methylation:How to deal with unpredictable conditions until the next generation and after. Mol Ecol 2010;19:1283-95. [CrossRef]
59. Qosim WA, Yuwariah Y, Hamdani JS, Rachmadi M, dan Perdani SM. Effect of ethyl methane sulphonate mutagent on shoot regeneration in two genotypes derived from purwakarta and pandeglang. J Hortic 2015;25:9-14. [CrossRef]
60. Pratiwi, P. Analysis of Multiple Genetic Variations Population of Globba leucantha Miq. In:West Sumatra with Random Amplified Polymorphic DNA (RAPD) (in Indonesia). Thesis. Indonesia:University Andalas;2012.
61. Hussain H, Nisar M. Assessment of plant genetic variations using molecular markers:A review. J Appl Biol Biotech 2020;8:99-109. [CrossRef]
62. Shah D, Kamili AN, Wani AA, Nazir N, Sajad N, Khan I, et al. Mutagenic action of ethyl methanesulphonate (EMS):A review. J Res Dev 2016;16:63-8.
63. Viana VE, Pegoraro C, Busanello C, Costa de Oliveira A. Mutagenesis in rice:The basis for breeding a new super plant. Front Plant Sci 2019;10:1326. [CrossRef]
64. Said WM, Ehsan NO, Khalifa NS. Comparative study of three species of Malvatheca (Bombacoideae and Malvoideae (Malvaceae sensu lato) using morphological, anatomical and RAPD-PCR analyses. Adv Environ Biol 2013;7:415-26.
65. Bani PW, Daryono BS, Purnomo P. Inter simple sequence repeat molecular markers to determine the resistance of maize plants to downy mildew. J Fitopatologi 2017;13:127-35. [CrossRef]