1. INTRODUCTION
Auxin is a phytohormone that induces cell division and elongation for plant developmental processes including gametogenesis, embryogenesis, seedling growth, vascular patterning, and flower development [1]. Plants can produce indole-3-acetic acid (IAA) auxin, to initiate primary and lateral root formation, regulate leaf morphogenesis, involve in plant-pathogen interactions and the defense mechanism of plants, and play in stress response by interaction with other plant hormones as abscisic acid, cytokinin, salicylic acid, gibberellin, and jasmonic acid, indispensable during the response mechanism to stress factors, such as drought and salinity [2]. Exogenous IAA from IAA producing bacteria, for example, rhizobacteria, could help plants’ tolerance to stress by improving root architecture, increasing membrane permeability for water diffusion into the cell [3]. Most endophytes are plant growth promoting microorganisms which can produce IAA. There are five major tryptophan dependent pathways for IAA synthesis in microorganisms as follows (i) indole-3-acetamide (IAM) pathway (L-Trp → IAM → IAA), (ii) indole-3-pyruvate (IPyA) pathway (L-Trp → IPyA → indole-3-acetaldehyde (IAAld) → IAA) with indole-3-lactic acid (ILA) and indole-3-ethanol (IOL) that are reversible compounds of IPyA and IAAld, respectively, (iii) tryptamine (TAM) pathway (L-Trp → TAM → IAAld → IAA), (iv) indole-3-acetonitrile (IAN) pathway (L-Trp → IAN → IAA or L-Trp → IAN → IAM → IAA), (v) tryptophan side chain oxidase pathway (L-Trp → IAAld → IAA) [4]. IAA production by microorganisms is recent interest because different strains have showed different biosynthesis pathways, which may affect plant growth and cell development.
The endophytic bacterium, Micrococcus luteus 4.43, isolated from Jerusalem artichoke, exhibited growth promotion under drought stress through IAA activity [5]. This strain demonstrated highly efficient IAA production in biotic nutrient broth (NB) and abiotic (NB with polyethylene glycol) conditions with 1 mg/ml of L-tryptophan as a precursor. Therefore, the bacterium could be a natural resource producing high yield IAA for agricultural biotechnology. Then, the bacterial IAA could be applied in agriculture directly using the supernatant from culture or developed into pellets by immobilization, encapsulation, etc., for controlled IAA release into the soil and for promoting plant growth. The aims of this research were investigation of the optimum conditions for high IAA production from M. luteus 4.43 and the determination of IAA stability against heat and light for long-term shelf life. In addition, the pathway of IAA synthesis of M. luteus 4.43 determined the intermediate compounds present during IAA production using HPLC to understand the relationship between IAA producing endophytic bacteria and plant hosts.
2. MATERIALS AND METHODS
2.1. Bacterial Strain and Cultivation
The strain 4.43 was identified using 16S rRNA gene of M. luteus 4.43 with accession number MH973230. The bacterium was grown on nutrient agar (HiMedia, India) at 30°C for 24 h, and also in NB with 1 mg/ml of L-tryptophan (ACROS organics, Belgium) at 30°C, 48 h with agitation at 150 rpm for IAA production.
2.2. Determination of Optimum Conditions for IAA Production
Overnight culture of M. luteus 4.43 was centrifuged to collect the cell pellets which were resuspended in 0.85% (w/v) NaCl and adjusted to 106 CFU/ml. The bacterial suspension (0.5% v/v) was transferred into NB with 1 mg/ml of L-tryptophan (L-Trp), then incubated at 30°C with agitation at 150 rpm for 48 h. The supernatant was collected by centrifugation at 10,000 rpm for 10 min after which IAA content was determined using colorimetric method. The optimum conditions of IAA synthesis were investigated in an initial bacterial concentration (103–108 CFU/ml), L-tryptophan concentration (0–5 mg/ml), temperature (25–40°C), pH (6–10), and incubation time (0–96 h).
2.3. Characterization of IAA Stability
M. luteus 4.43 grew in NB with 3 mg/ml of tryptophan at 30°C for 48 h and then the culture was centrifuged at 8000 rpm for 10 min to collect the supernatant (cell free culture). One part of the supernatant was adjusted to pH 2.0 with 5M HCl and then centrifuged to collect the supernatant which was mixed with ethyl acetate in the ratio of 1:1. After that, the mixture was evaporated to yield crude IAA extracts [6]. Two samples, the supernatant (cell free culture without extraction) and IAA extracts, were examined for stability against various temperatures, and light conditions to determine shelf-life. For temperature test, the samples were heated at autoclave condition (121°C, 15 min) and kept at 4°C [7]; the samples (without heat) were kept at 4°C and room temperature (∼30°C) in dark condition [8]. In another experiment, the samples were exposed to light at 1000 lux for 12 h per day at room temperature [8]. All samples were collected at various times until 60 days to determine IAA content.
2.4. Colorimetric Determination of IAA
The reaction comprised of sample and Salkowski’ reagent (50 ml of 35% HClO4+1 ml of 0.5 M FeCl3) at a ratio of 1:1, incubated in the dark for 30 min. Absorbance of the reaction was detected at 530 nm [9]. The IAA content was calculated from IAA standard curve and expressed as μg IAA per unit of optical density [10].
2.5. Detection of Intermediate Compounds During IAA Biosynthesis
The supernatant, from the culture of M. luteus 4.43 grown in NB with 3 mg/ml of tryptophan at 30°C, was collected at 0–96 h and transferred into a centrifugal filtration using 3-kDa cutoff membrane filters. The filtrate samples (10 µl) were passed through 0.2 µm filter membrane and injected into a C8 column (Symmetry 4.6 × 150 mm, Inertsil®) using gradient elution for separation. The mobile phase A: B was at 80:20% [mobile Phase A consisted of acetic acid and deionized water in the ratio of 2.5:97.5% (pH 3.8); mobile Phase B consisted of acetonitrile and deionized water in the ratio of 80:20%]. The flow rate was 1 ml/min with HPLC coupled with fluorescence detector (Shimadzu LC-20A HPLC system) at a wavelength of 280 nm for excitation and 350 nm of emission [11]. The number of intermediate compounds was calculated from a calibration curve of five indole compounds as standards, such as TAM, IAM, ILA, IAN, IAA, and L-Trp (Sigma-Aldrich, Germany).
2.6. Statistic Analysis
Statistical analysis was performed using Statistix 8.0 and Fisher’s least significant difference (LSD) at 99% (P < 0.01) was used to analyze significant difference in all experiments.
3. RESULTS AND DISCUSSION
3.1. An Optimum Condition of IAA Production from M. luteus 4.43
The endophytic bacterium, M. luteus 4.43, was isolated from Jerusalem artichoke and demonstrated IAA production (a phytohormone). To increase IAA yield, an initial inoculum size was determined in different cell number for inoculation. Cell numbers at 106–108 CFU/ml showed the highest IAA production [Figure 1a]; IAA content was decreased in inoculums with the lower cell numbers. The correlation between the cell number and IAA production was significantly positive [Figure 1a]. The tryptophan concentration was an important factor for IAA production as it was a precursor of IAA synthesis. The more tryptophan in the medium, the greater IAA was detected with high positive correlation [Figure 1b]. Thus, tryptophan was used at 5 mg/ml in the next experiment with 106 CFU/ml of inoculum. The optimum temperature and pH of IAA production were 30°C at pH 7.0 [Figure 1c and d]. These factors showed negative correlation; this meant that temperature and pH were related to bacterial growth leading to an effect on IAA production. Finally, the optimum duration of culture was determined to achieve the highest IAA. The correlation curve was positive; at 72 h, the bacterium produced 784 ± 44.81 mg/ml of IAA production [Figure 1e].
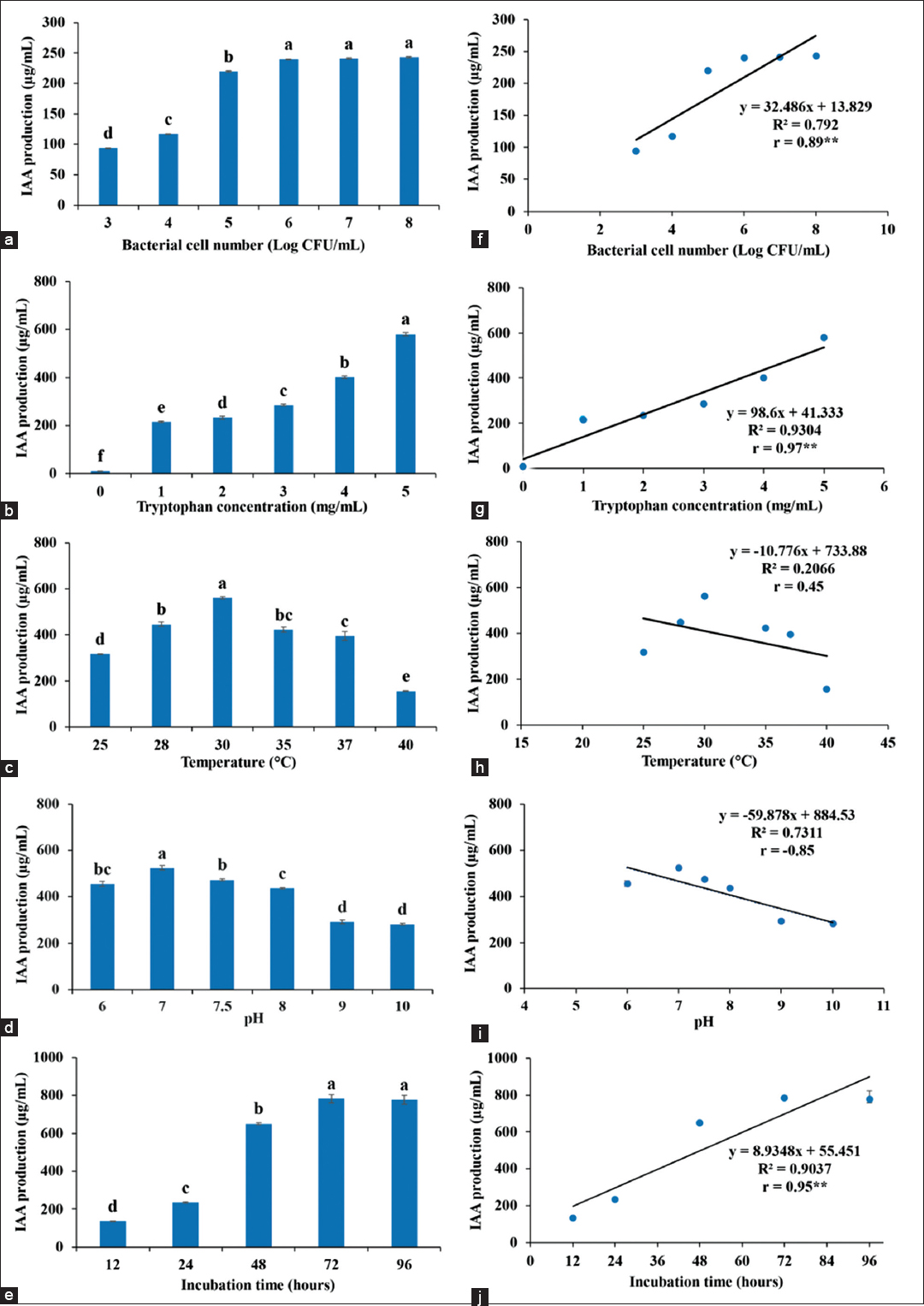 | Figure 1: Factors affect IAA production of Micrococcus luteus 4.43, (a) inoculum size, (b) tryptophan concentration, (c) temperature, (d) pH, (e) time; and correlation of each factor (f-j) to IAA production was shown in parallel graph with r value. ** means significant difference in data at P < 0.01. [Click here to view] |
Many bacteria were capable of IAA production in the range of 10–200 g/ml, such as Bacillus [12], Enterobacter [13], Pseudomonas [14], Azospirillum [15], Agrobacterium, and Rhizobium [16], most of which are plant growth promoting bacteria. The genus Micrococcus was reported as bacterial endophytes of rice seeds that were IAA producing bacteria, Micrococcus yunnanensis RWL-2 and M. luteus RWL-3 which produced IAA in range of 11.50 ± 0.77 μg/ml–38.80 ± 1.35 μg/ml [17]. Similarly, M. luteus 4.43 was an endophyte of Jerusalem artichoke that was able to produce high IAA production (784 ± 44.81 μg/ml) in this work. However, Enterobacter sp. demonstrated the highest IAA production at 3378–3477 μg/ml [6]. Comparison of fungal producing IAA concluded that endophytic fungus, Colletotrichum fructicola, was a good strain for high IAA production at 1205.58 ± 151.89 μg/ml [18]; moreover, ectomycorrhizal fungi yielded IAA production at 54.56 ± 2.21 μg/ml [19]. Fungi are capable of producing IAA with high production depending on strains of fungi, but they take longer time for cultivation (26–30 days). The main factor for IAA production was tryptophan, as IAA biosynthesis of microorganisms requires tryptophan precursor via different intermediate pathways [4]. IAA production began at log phase through stationary phase of bacterial growth to achieve high IAA production [Figure 1e]. Temperature and pH were factors of microorganisms’ growth but they were not directly effects on IAA production according to negative correlation between IAA production and temperature or pH. The rhizospheric bacteria showed the optimum temperature at 30°C and the optimum pH at pH 7–9 for IAA production. The suitable tryptophan was in range of 0.05–1.5%. In addition, the nutrients (carbon and nitrogen sources) had effect on IAA production; the mannitol and ammonium nitrate were added into the medium for high IAA production [20].
3.2. Bacterial IAA Stability to Heat and Light
The samples of supernatant and IAA extracts were used to test stability. The supernatant obtained IAA was stable at room temperature and at 4°C in the dark for 60 days with more than 50% of residual IAA activity [Figure 2a]. It also was tolerant to heat (121°C, 15 min) when kept at room temperature in the dark for 60 days, retaining more than 50% of residual IAA. The results of the supernatant showed the same results of IAA extracts, except that the IAA extracts lost 70% after 30 days of storage at room temperature [Figure 2b]. For light exposure, the supernatant and IAA extracts showed long-term stability (30 days) of storage at room temperature. After 30 days, more than 70% loss of IAA activity was observed in both samples.
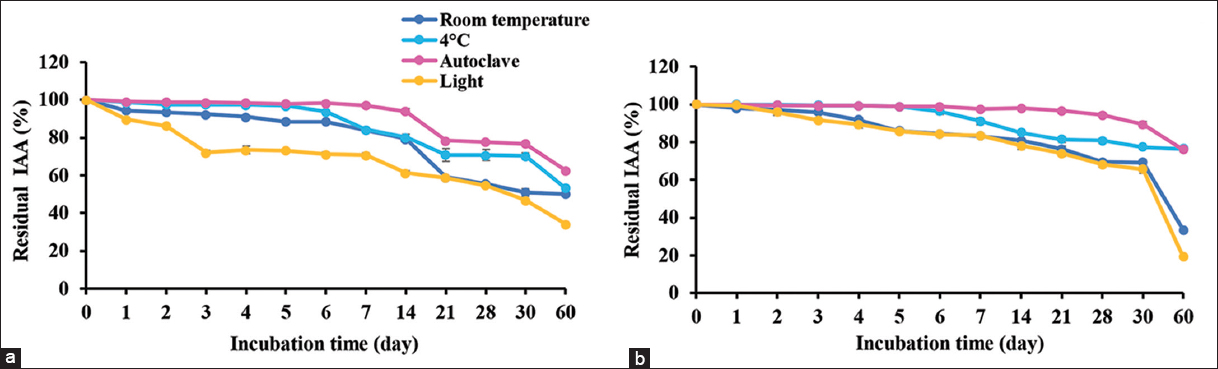 | Figure 2: Stability of IAA from supernatant of cell culture (a) and crude IAA extracted from supernatant (b) that were kept at room temperature and 4°C; also tested for heat resistance at autoclave condition and light exposure at 1000 lux. The residual IAA was determined in various times for shelf life. [Click here to view] |
M. luteus 4.43 produced IAA, releasing into the cell culture, called the supernatant, which tolerated heat (autoclave condition) and showed the same stability with unheated samples. Therefore, the heated sample may be a better IAA preparation for direct usage as its sterility reduces contamination of bacteria and some proteins. Moreover, the IAA extracts with heat treatment showed the best storage longevity in the dark at room temperature. The results demonstrated the stability of IAA against heat at similar levels to synthetic IAA, which has been confirmed as stable to autoclaving within the ordinary pH range, and also to oxygen in the air under the usual aeration or shaking condition [8]. For light exposure, the lower light intensity (1000 lux) had little effect on IAA for the first 30 days of storage, but IAA activity dropped continuously after 30 days. The light intensity is very important criteria for IAA storage including light spectrum. The blue light did greater damage to IAA compared with red light at the same intensity [8]. The results confirmed that IAA produced from M. luteus 4.43 possessed good properties as commercial synthetic IAA. The company instructions on preparation of synthetic IAA suggest sterilization by autoclave or filtering through 0.2 μm filter membrane, then being kept in the dark at 4°C (Gold Biotechnology, USA). Further study should explore the formulation of IAA for optimum user-friendliness and efficiency, for example, IAA encapsulation, IAA immobilization with agricultural waste or biofertilizer with IAA compound.
3.3. Intermediate Compounds during IAA Biosynthesis
The calibration curves showed linear lines of response on indole compound concentrations [Figure 3] and the linear regression equation with coefficient of determination showed in Table 1 [r2 ≥ 0.996]. The indole compounds standard exhibited retention times as follows; 5.34, 6.47, 14.42, 17.48, 24.91, and 37.92 min for L-Trp, TAM, IAM, ILA, IAA, and IAN, respectively [Figure 4a]. The retention times of each compound were different from the literature [11]; this was caused by condition and pH of mobile phase. The medium control without bacterial inoculation was a negative control [Figure 4b] and the supernatant of M. luteus 4.43 obtained five intermediate compounds (TAM, IAM, ILA, IAA, and IAN) corresponding to the retention time of the standards [Figure 4c and d]. The intermediate compounds were quantified from calibration curves as shown in Table 2. TAM was detected after 12 h and had the greatest amount at 96 h; while ILA showed highest levels at 12 h and then constant amounts not over 10 μg/ml. IAN quantities were lowest compared to all intermediate compounds and in parallel to IAM were slightly increased along incubation time. For IAA production, the bacterium produced straight after inoculation was in small quantities, producing the highest amounts after 48 h. Thus, the bacterium mainly used IAN pathway for IAA synthesis. The high ILA was detected in the culture from catalyzing L-Trp into IPyA using aromatic amino acid aminotransferase; and IPyA had reversible transamination reaction between IPyA and ILA. According to Numponsak et al. [18] and Kumla et al. [19], the peaks of IPyA and indole-3-ethanol (IOL) had the retention time after the peaks of IAA. However, no peak was observed between IAA and IAN peaks in this work; the results implied that there was no compound during retention time of 26–36 min. Moreover, IPyA was able to reverse into L-Trp through aminotransferase which showed high levels of L-Trp at 48 h and 96 h in this experiment. The results implied that L-Trp was metabolized to IPyA and ILA, but did not change to IAA similar to IAA production of Pseudomonas putida [11]. Likely, TAM was detected in high amounts due to metabolization of L-Trp through decarboxylase enzyme; but no intermediate compound of TAM pathway (IOL) was observed. This implied that the bacterium did not use neither IPyA nor TAM pathway for IAA synthesis. IAN and IAM were detected in low amounts; both compounds were able to convert into IAA directly. After 12 h, the amount of IAN was constantly low and IAM was slightly increased; with IAA the converse was true. This indicated that IAN was changed into IAA and also changed into IAM which was further converted to IAA, leading to high levels of IAA synthesis.
Table 1: Calibration curves parameters of indole compounds standard.
| Compounds | Retention time (min) | Linear regression equation | Coefficient of determination (r2) |
|---|---|---|---|
| TAM | 6.47±0.276 | y=383857×−194495 | r2=0.9960 |
| IAM | 14.42±0.509 | y=1E+06×+37543 | r2=0.9962 |
| ILA | 17.48±0.387 | y=577201×−7731.7 | r2=0.9967 |
| IAA | 24.91±0.697 | y=596728×+39454 | r2=0.9998 |
| IAN | 37.92±0.631 | y=2E+06×−107785 | r2=0.9957 |
TAM: Tryptamine, IAM: Indole-3-acetamide, ILA: Indole-3-lactic acid, IAA: Indole-3-acetic acid, IAN: Indole-3-acetonitrile.
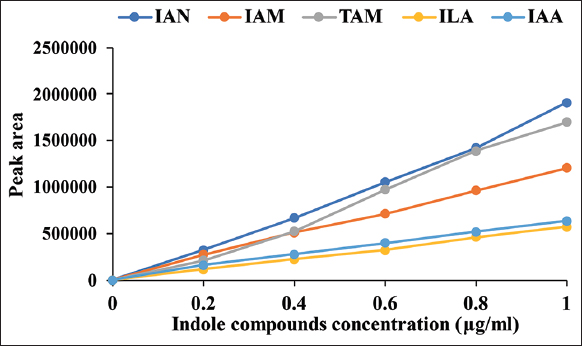 | Figure 3: Calibration curves of indole compounds standard exhibited peak area of each compound concentrations. [Click here to view] |
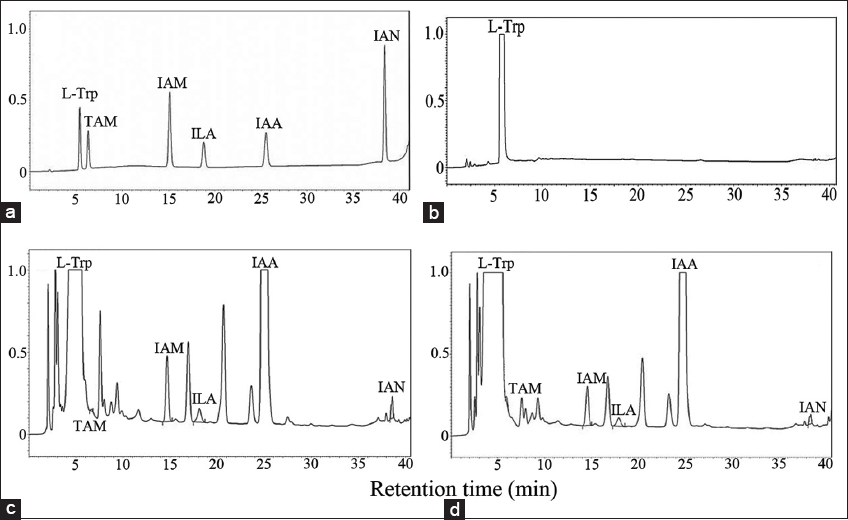 | Figure 4: Chromatogram of intermediate compounds during IAA production of Micrococcus luteus 4.43 by HPLC technique. Indole compounds standard (a), cell-free culture in NB with tryptophan at 0 h (b), at 48 h (c), at 96 h (d). L-Trp: L-tryptophan, TAM: Tryptamine, IAM: Indole-3-acetamide, ILA: Indole-3-lactic acid, IAA: Indole-3-acetic acid, and IAN: Indole-3-acetonitrile. The analyses were performed by triplicate. [Click here to view] |
Table 2: Amounts of intermediate compounds during IAA biosynthesis with tryptophan.
| Type | Amounts of each compound in various times (mg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 h | 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | 96 h | |
| TAM | 0 | 0 | 0 | 15.72±0.11 | 16.45±0.10 | 17.05±0.77 | 20.49±2.78 | 16.45±0.30 | 36.61±1.61 |
| IAM | 0 | 0.50±0.11 | 4.58±0.55 | 4.08±0.19 | 4.70±0.38 | 5.59±0.44 | 7.34±0.06 | 7.91±1.18 | 8.86±0.19 |
| ILA | 0 | 1.43±0.22 | 37.36±0.97 | 3.11±1.43 | 4.47±0.21 | 4.24±0.06 | 4.69±0.10 | 10.48±9.42 | 5.11±0.37 |
| IAN | 0 | 1.12±0.16 | 9.60±0.21 | 2.08±0.36 | 1.82±0.02 | 2.06±0.13 | 1.80±0.01 | 2.66±0.05 | 2.86±0.03 |
| IAA | 0 | 12.69±1.54 | 335.41±2.30 | 505.93±10.04 | 950.30±2.45 | 826.12±12.34 | 1066.02±15.85 | 1001.71±39.34 | 1029.12±7.63 |
TAM: Tryptamine, IAM: Indole-3-acetamide, ILA: Indole-3-lactic acid, IAA: Indole-3-acetic acid, IAN: Indole-3-acetonitrile.
Bacteria obtained the genes and enzymes involved in IAA biosynthesis with tryptophan as a precursor through five pathways as described in Spaepen et al. [4]. The IAM, IAN, and IPyA pathways are considered the major IAA biosynthesis pathways in bacteria [21], for example, IPyA pathway was found in plant growth promoting bacteria, Enterobacter cloacae and Bacillus amyloliquefaciens FZB42 [22]; IAM was found in Streptomyces spp. [23] and IAN was found in Agrobacterium spp. and Rhizobium spp. which were changed IAN into IAM and IAA [16], while Bradyrhizobium japonicum produced IAA through either IAN or IAM [24]. TAM pathway is common for IAA synthesis in plant and fungi [25]. However, Azospirillum brasilense used TAM and IAN for IAA synthesis [26]. In this work, the evidence showed IAA biosynthesis of M. luteus 4.43 through IAN pathway. The conversion steps possibly involve changing L-Trp into indole-3-acetaldoxime as another intermediate compound before further change to IAN and IAA by nitrilase [25]; alternatively, L-Trp was changed to IAN and then converted to IAM by a nitrile hydratase and an amidase into IAA [27].
4. CONCLUSION
The endophytic bacterium, M. luteus 4.43 produced very high IAA levels (784±44.81 mg/ml) depending on the initial cell number of inoculum, L-tryptophan concentration, and incubation time; but it had a negative correlation to pH and temperature. Either form of IAA between the supernatant (obtained IAA) and IAA crude extract showed good stability to heat (autoclave), light (1000 lux) and long storage life at 4°C and room temperature in the dark for up to 2 months. This strain exhibited TAM, ILA, IAM, and IAN as intermediate compounds of IAA synthesis during culture in medium with tryptophan. According to the amount of IAA, it was concluded that this strain catalyzed L-Trp into IAN which then changed directly to IAA; moreover, it possibly also converted IAN into IAM and IAA. Hence, M. luteus 4.43 produced IAA through IAN pathway mainly.
5. ACKNOWLEDGMENTS
This research was supported by the Fundamental Fund of Khon Kaen University from the National science, research and innovation fund; and also, was supported by the Protein and Proteomics Research Center for Commercial and Industrial Purposes, Khon Kaen University, Thailand.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, analysis, and interpretation of data; took part in drafting the article.
7. CONFLICTS OF INTEREST
The authors declare that there is no conflicts of interests.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
No additional or supplementary data files associated with the manuscript.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 2010;61:49-64. [CrossRef]
2. Zhang Y, Li Y, Hassan MJ, Li Z, Peng Y. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol 2020;20:150. [CrossRef]
3. Gamalero E, Glick BR. Recent advances in bacterial amelioration of plant drought and salt stress. Biology (Basel) 2022;11:437. [CrossRef]
4. Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 2007;31:425-48. [CrossRef]
5. Namwongsa J, Jogloy S, Vorasoot N, Boonlue S, Riddech N, Mongkolthanaruk W. Endophytic bacteria improve root traits, biomass and yield of
6. Zhang BX, Li PS, Wang YY, Wang JJ, Liu XL, Wang XY,
7. Nissen SJ, Sutter EG. Stability of IAA and IBA in nutrient medium to several tissue culture procedures. HortScience 1990;25:800-2. [CrossRef]
8. Yamakawa T, Kurahashi O, Ishida K, Kato S, Kodama T, Minoda Y. Stability of indole-3-acetic acid to autoclaving, aeration and light illumination. Agric Biol Chem 1979;43:879-80. [CrossRef]
9. Tsavkelova EA, Cherdyntseva TA, Botina SG, Netrusov AI. Bacteria associated with orchid roots and microbial production of auxin. Microbiol Res 2007;162:69-76. [CrossRef]
10. Sasirekha B, Shivakumar S, Sullia SB. Statistical optimization for improved indole-3-acetic acid (iaa) production by
11. Szkop M, Bielawski W. A simple method for simultaneous RP-HPLC determination of indolic compounds related to bacterial biosynthesis of indole-3-acetic acid. Antonie Van Leeuwenhoek 2013;103:683-91. [CrossRef]
12. Lim JH, Kim SD. Synergistic plant growth promotion by the indigenous auxins-producing PGPR
13. Ryu RJ, Patten CL. Aromatic amino acid-dependent expression of indole-3-pyruvate decarboxylase is regulated by TyrR in
14. Howden AJ, Rico A, Mentlak T, Miguet L, Preston GM.
15. Castro-Guerrero J, Romero A, Aguilar JJ, Xiqui ML, Sandoval JO, Baca BE. The hisC1 gene, encoding aromatic amino acid aminotransferase-1 in
16. Kobayashi M, Suzuki T, Fujita T, Masuda M, Shimizu S. Occurrence of enzymes involved in biosynthesis of indole-3-acetic acid from indole-3-acetonitrile in plant-associated bacteria,
17. Shahzad R, Waqas M, Khan AL, Al-Hosni K, Kang SM, Seo CW,
18. Numponsak T, Kumla J, Suwannarach N, Matsui K, Lumyong S. Biosynthetic pathway and optimal conditions for the production of indole-3-acetic acid by an endophytic fungus,
19. Kumla J, Suwannarach N, Matsui K, Lumyong S. Biosynthetic pathway of indole-3-acetic acid in ectomycorrhizal fungi collected from Northern Thailand. PLoS One 2020;15:e0227478. [CrossRef]
20. Mohite B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutr 2013;13:638-49. [CrossRef]
21. Duca D, Lorv J, Patten CL, Rose D, Glick BR. Indole-3-acetic acid in plant-microbe interactions. Anton Leeuw 2014;106:85-125. [CrossRef]
22. Idris EE, Iglesias DJ, Talon M, Borriss R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by
23. Manulis S, Haviv-Chesner A, Brandl MT, Lindow SE, Barash I. Differential involvement of indole-3-acetic acid biosynthetic pathways in pathogenicity and epiphytic fitness of
24. Torres D, Benavidez I, Donadio F, Mongiardini E, Rosas S, Spaepen S,
25. Patten CL, Glick BR. Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 1996;42:207-20. [CrossRef]
26. Carreño-Lopez R, Campos-Reales N, Elmerich C, Baca BE. Physiological evidence for differently regulated tryptophan-dependent pathways for indole-3-acetic acid synthesis in
27. Zhao Y. Auxin biosynthesis:A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol Plant 2012;5:334-8. https://doi.org/10.1093/mp/ssr104 PMid:22155950 PMCid:PMC3309920