1. INTRODUCTION
The environment protection agency (EPA) plan calls for advanced biofuel volume mandates to be set at 5.82 billion gallons in 2023, 6.62 billion in 2024, and 7.43 billion in 2025. The average American uses 2.5 gallons of oil, 8.86 pounds of coal, and 246 cubic feet of natural gas every day [1]. The world is eagerly anticipating a time when all energy sources will be used. Fuels are crucial since they are the main source of energy. Over the past few decades, concern over the depletion of traditional fuel supplies and a desire for biofuel production has grown. The products generated by using biomass are using a carbon-neutral path and absorb the maximum amount of CO2 [2]. Biodiesel is nontoxic and perishable and is created by combining alcohol with edible fat, animal fat, or used cooking oil. Considering the food issues, microalgae gain the utmost attention to be used as biofuel producers. According to the study, biomass-based biofuels offer excellent potential to address energy scarcity in a sustainable manner [3]. The environmentally beneficial method of producing biodiesel has attracted the interest of scientists worldwide [4]. The effectiveness and cost-reduction of microalgal biodiesel synthesis continue to be major barriers to its optimal development because it is a sequential process [5]. The costliest of the four production processes is cultivation (>40% of the overall cost). Among the four production methods, cultivation stands out as the most expensive (>40% of the total expenses). To achieve a significant biomass concentration, it is essential to gather microalgae [6]. Consequently, substantial quantities of water need to be acquired from the production procedures, which entails financial expenditure and energy consumption. It is estimated that around 30% of the overall production cost could be attributed to the expenses related to harvesting [7]. To accomplish energy-effective microalgae harvesting, novel functional materials have been created due to the substantial energy consumption during these harvesting methods. Harvesting with bare nanoparticles (NPs) has become a popular method in recent years for separating cyanobacteria, microalgae, and algae from biofuel perspectives [8]. The ability of NPs to influence cell fate, cause or stop mutations, start cell–cell communication, and modify cell structure is largely determined by processes at the nano-bio interface. Although adding nano-additives to microalgae cultures has certain benefits. There are a number of factors relating to the properties and concentration of the NPs that must be taken into account. Recently, nanomaterials primarily based on magnetic activity have been introduced as quick, easy, and effective techniques for microalgae harvest. NPs have special physicochemical features that have the potential to improve agricultural products, food processing, fortification, and packaging. In addition to shielding plants against biotic and abiotic challenges, many metal NPs (e.g., Cu, Fe, Zn, Mg, Mn, Se, etc.) also serve as micronutrients for crop plants [9]. A thorough investigation is needed to determine the most crucial factor relating to the toxic effects of NPs on various microalgae species, specifically from substances such as oxides of iron, zinc oxide, zero-valent iron (ZVIs), aluminum oxide, graphene oxide, and titanium dioxide, etc. Different species such as algae, microalgae nitzschia, chlorella, and schizochytrium have suitable potential for biodiesel production. The main objective of the review is to provide a thorough understanding of the mechanism of NPs, impacts of NPs on microalgae and their real-world use, and potential future research. The current review synthesized the previously published work, which includes different channels of NP inoculation with microalgae. Future applications of engineered and non-engineered nanomaterials to strengthen microalgae’s ability for biodiesel production are also taken into consideration.
2. NP INCORPORATION IN ALGAL CELLS
Nanoscience primarily deals with the synthesis, characterization, exploration, and exploitation of nanostructured materials. By utilizing the methodological facts, manipulation, and control of matter on the nanoscale, known as Nanotechnology. According to their sizes, NPs can be classified into different dimensions - 0, 1, 2, and 3 [10]. Depending on the chemical composition, morphological structure, and origin, particles can be further classified.
A range of nanoparticle sizes, from 5 to 100 nm, have been reported in the literature for use in biofuel generation [11]. The effect of NPs on algae has been extensively studied during the last 20 years [12]. Prokaryotic cyanobacteria and eukaryotic micro- and macroalgae make up the enormous and varied group of microorganisms that produce oxygen through photosynthetic processes. Algae are thought of as a renewable bioenergy source due to their capacity for photosynthetic growth [13]. Algae have long been recognized for their promise in the fields of carbon sequestration, bioremediation, and wastewater treatment. They are a priceless source of important substances such as polyunsaturated fatty acids, carotenoids, carbohydrates, proteins, amino acids, astaxanthin, glutathione, phycotoxins, etc., that are used in medicine, bioactive compounds, and other high-value products [14]. Several methods exist for introducing NPs into algal cells, including passive uptake, chemical transformation, electroporation, sonication, and microinjection. The choice depends on nanoparticle type, algae species, and intended application. Passive uptake involves natural absorption over time, while chemical transformation uses functionalization to enhance uptake. Electroporation creates temporary pores, and sonication disrupts the cell membrane temporarily. Microinjection involves direct injection into algal cells using micropipettes or microfluidic devices [83].
Studies have suggested that NPs may be used because of their high biomass yield, ease of synthesis, stability in nature, easy removal, and reused. It is critical to meticulously study the mechanisms of NP internalization in algal cells and subsequent toxicity for risk assessment of NPs in the aquatic ecosystem. For NPs to accumulate, they must first be adsorbed onto the functional groups of the algal cell wall. The charged NPs (positive or negative) in the nearby environment or medium will stick to the negatively charged cell surface through electrostatic contact [15].
3. MECHANISM OF NPs UPTAKE IN MICROALGAE
Modification and uptake of NPs are understood in plant and animal cells [16], while internalization processes in cyanobacteria and algae remain largely speculative. Interactions involve shade, ion release, adsorption, absorption, and cell-wall rupture [17]. Algal cell walls, like in green algae, comprising cellulose, homogalacturonan, etc. [18,19], are initial contact points. NPs adhere to functional groups on the wall for accumulation. Blue–green algae release Extracellular Polymeric Substances (EPS) in response to NPs, altering EPS composition [20]. Algal wall proteins tied to polysaccharides create functional groups [21]. EPS, with exopolysaccharides and glycoproteins, aids uncharged NP adsorption, and broken cell walls enable NP diffusion [22]. EPS influences NP adsorption due to electrostatics, bonds, and hydrophobicity. As per the study, an activated sludge derived-extracellular polymeric substance (ASD-EPS) was added to Chlorella vulgaris culture medium, and 87.24% harvesting efficiency was achieved [84]. Similarly, the highest flocculation effectiveness reached 91.8% when employing 30 mL/L of EPS (with an EPS-to-biomass ratio of 0.40 g EPS/g). This EPS was extracted using formaldehyde-NaOH [85].
Cell wall thickness and composition impact NP internalization. Algal walls differ from higher plants [23]. NPs cross membranes via diffusion or unknown protein-mediated systems. Hydrophobic traits are necessary, largely unobserved in NPs.
Proteins on the membrane act as carriers, with positive NPs penetrating cells more than negatives in the protein corona. pH impacts protein-NP affinity due to electrostatic forces; positively charged proteins and lower pH favor binding [24]. NP protein corona is observed in animals, not well in blue-green algae or cyanobacteria. Ion transfer occurs actively and passively across membranes. Integral membrane proteins, ionophores, or carriers are vital for facilitated diffusion, aiding ion transport [25].
The process of endocytosis causes NPs to be engulfed in membrane invaginations, followed by their budding and pinching off to create endocytic vesicles, which are then transported to specialized intracellular sorting/trafficking compartments [26]. The five primary endocytosis mechanisms – phagocytosis, clathrin-mediated endocytosis, caveolin-mediated endocytosis, clathrin/caveolae-independent endocytosis, and micropinocytosis were broadly discussed for plant cells [27]. The incorporation of metallic and non- metallic NPs in various microalgal species has been demonstrated in numerous investigations. It was investigated that at the microalgae cell wall, zero-valent iron NPs (nZVI) electrochemically interact with exopolymeric substances (EPS), and the microalgae then internalize these NPs through endocytosis [28]. The endocytotic mechanism of NPs uptake in algae and cyanobacteria is mostly unexplored. Although, it has been reported that the internalization of iron oxide NPs through endocytosis pathways improved the lipid yields in Chlorella pyrenoidosa [29]. The introduction of La3+ expedited endocytic processes in E. gracilis, enhancing nutrient uptake (glucose, macro/micro elements). In adduition, La3+ elevated paramylon production in E. gracilis along with fatty acid methyl ester (FAME) content [86]. According to reports, the efficiency of biodiesel production in Scenedesmus obliquus was enhanced by 80% through the utilization of an immobilized enzyme system. This system involved Candida rugosa lipase being bound to magnetic nickel ferrite NPs (NiFe2O4) that were coated with graphene oxide (GO) [87].
Cellular structure, wall composition/thickness, membrane characteristics, and particle properties affect NP uptake and accumulation, requiring further study [30]. NP-cell interactions can be influenced by variations in NP qualities or membrane properties. NPs are increasingly used in commercial and personal care products, entering aquatic environments [31]. Agglomeration of NPs can synergistically impact aquatic ecosystems, alone or with other pollutants like metals [31]. Algae represent aquatic ecosystems, necessitating close observation of NP mechanisms due to their importance [Figure 1].
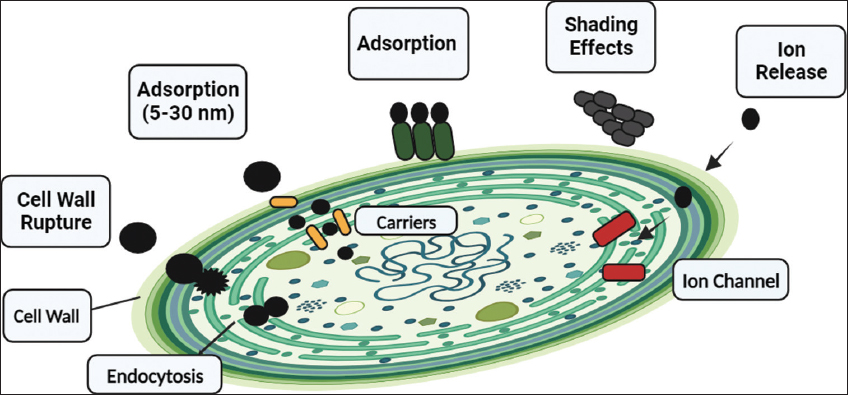 | Figure 1: Proposed interactions between nanoarticles and microalgae include endocytosis, ion release (absorption through ionic channel), shading impact, adsorption (which prevents ionic exchange), and absorption by pore pathway. [Click here to view] |
4. EFFECTS OF NPs ON MICROALGAE
A thorough assessment is given on the impact of various NPs on the metabolic pathways of microalgae, light conversion, toxicity, cellular damage, and accumulation of intracellular chemicals. It is advised to focus on process optimization for better microalgae development. In order to promote the energy-efficient commercialization of microalgae for a wide range of application channels, it is intended that this review would stimulate research on the effective application of NPs to microalgae cultivation and harvesting.
4.1. Effect of NPs on Microalgae Growth
To investigate their impact on the growth rate, biomass production, and accumulation of intracellular chemicals, metal NPs have been incorporated into microalgae culture at various concentrations. To enhance the development and lipid production of photosynthetic microalgae cells, these substances have been utilized as nutritional supplements.
The impact of different concentrations of ZnO NPs on the growth of Chlorella sp. was measured, and studies revealed that at a lower concentration of 50 mgL-1, no aggregation was found, but at 100 mgL-1 accumulation of microalgae was observed [32]. The significant slowdown in growth rate is inversely related to the rise in NPs concentrations. Metal salt (Cu2+), nano-metal (nano-Cu), and nano-metal oxide (nano-CuO) prohibit the growth of marine phytoplankton Skeletonema costatum and Nitzschia closterium. It has been observed that Cu2+ and nano-Cu EC50 values from 0.356 to 0.991 mgL-1 and 0.663 to 2.455 mgL-1 affect the secretion of extracellular polymeric substances and amino acids in S. costatum and N. closterium [33]. Attheya ussuriensis (Bacillariophyceae), Chaetoceros muelleri (Bacillariophyceae), Heterosigma akashiwo (Raphidophyceae) and Porphyridium purpureum (Rhodophyceae) were exposed to two types of multiwalled silica nanotubes SNT-1 and SNT-2 for 96 h (7 days) and reported that the growth rate was affected for only C. muelleri and P. purpureum [34]. Scanning electron microscope observation of filamentous green microalgae Klebsormidium flaccidum showed morphological changes when treated with 100 mgL-1 multi-walled carbon nanotubes (CNT) for 48 h of contact [35].
Accordingly, based on the aforementioned investigations, some microalgae species were favored by lower nanoparticle concentrations, whilst larger concentrations had a negative impact on the density and growth of microalgae. The sensitivity of microalgae to NPs largely depends on the kind of nanoparticle and the species of microalgae, as well as other parameters such as culture media, pH, shape, and size of the NPs; hence, the limit of “low” or “high” concentrations cannot be defined. It has been proved that inoculation of NPs in several microalgal species like Chlorella, Scenedesmus, and Nannochloropsis, etc, results in an increase in total biomass, and accumulation of carbohydrate and lipid content.
4.2. Effect of NPs on Microalgae Biomass
Using NPs in the culture of microalgae is an applicable technique to customize biomass output and generating high-value products like biofuels. Chlorella, Scenedesmus, and Nannochloropsis spp. are affected by NPs in a way that causes an increase in total biomass and also improves the accumulation of carbohydrate and lipid content. Although the effects of NP treatment on the aforementioned types of algae were generally good, there may be variations in the way certain metabolites accumulate and how much of them are produced depending on the physiology of the various algae species. Da costa et al., reported that exposure to Cr2O3-NP dramatically reduced the amount of chlorophyll a in the cells and impeded culture growth of unicellular green alga Chlamydomonas reinhardtii. After 24 and 72 h of exposure, respectively, EC50 values of 2.05 ± 0.20 and 1.35 ± 0.06 gL-1 Cr2O3-NP were determined from assessments of cell density [36]. It is investigated the effects of copper and selenium nano aqua chelate carboxylated with citric acid on biomass accumulation on the green algae Chlorella vulgaris. In addition, by measuring chlorophyll a fluorescence, the effectiveness of the processes during the light stage of photosynthesis was calculated. Chlorella biomass increased by around 20% with the addition of 0.67–4 mg L-1 of Cu nano carboxylates, but after the 12th day of incubation, concentrations of 20–40 mg L-1 substantially reduced algal development. Se nanocarboxylates at concentrations of 0.4–4 mg L-1 also promoted the development of C. vulgaris, with a 40–45% rise in biomass [37]. Similar study carried out on microalgae Dunaliella salina by Hassanpour et al., Fe2WO6 nps synthesized by ultrasound method incorporated in the strain at 20, 40 and 80 ppm respectively. Result showed that on 80 ppm the level of lipid peroxidation was higher [58]. It has been reported that the optimum dosages of Fe3O4 and Y3Fe5O12 to harvest microalgal biomass in Chlorella vulgaris were 10 and 2.5 g/L, while the suitable pH values were 6.2 and 7.3, respectively. As the pH level dropped, Fe3O4 and Y3Fe5O12 nanoparticle harvesting efficiency rose. The experimental results also showed that Fe3O4 NPs could be isolated from flocs considerably more readily than Y3Fe5O12 under conditions of higher pH. When the pH of the floc reached 12.3, 62.9% of the Fe3O4 NPs could be detached from the aggregates [38]. Effects of nano Fe2O3 were studied on two species of Chlorella pyrenoidosa and Chlorella sorokiniana. At 20 mg L-1 concentration C. pyrenoidosa showed 33.75% enhancement in biomass on the other hand C. sorokiniana showed toxicity even on the lower dose [29]. Similar studies were conducted on Scenedesmus obliquus to improve microalgal growth, by using three NPs; CNT, nano Fe2O3 and MgO and it was determined that low dose 0–20 mg L-1 of nano Fe2O3 promoted the growth. It has been suggested that elevated nanoparticle exposure had a restricted impact on biomass productivity, potentially attributed to the inhibition of cell growth triggered by nanoparticle-induced reactive oxygen species (ROS) generation. Consequently, this led to a decrease in biomass and lipid production [40]. Studies indicate that the incorporation of SiC NPs along with xenon lamp illumination presents a hopeful approach for enhancing both microalgal growth and lipid accumulation. Under the conditions of an ideal SiC NPs concentration set at 150 mg/L and a photoperiod of 6:18 h, the Scenedesmus sp. attained a peak biomass concentration of 3.18 g/L [41]. Magnetic nano particles (MNPs) exhibited a remarkable ability to capture Nannochloropsis maritima, achieving a high harvesting efficiency of 99.5% when used at a dosage of 0.33 g MNPs/g of dry biomass. Furthermore, the harvesting performance was found to be relatively unaffected by pH values within the range of 5.0–9.0. The efficiency of magnetic harvesting depends on the interface interaction between microalgae and MNPs [42]. In another study, on Chlamydomonas reinhardtii, Ag+ ions are released from Ag NPs, and the way these dissolved Ag+ ions are distributed is a critical aspect affecting the toxicity of metallic NPs in aquatic ecosystems [39] [Table 1].
Table 1: Effects of NPs on microalgal biomass.
| Microalgal species | NP | Size (nm) | Concentration | Effect on growth (Biomass %) | References |
|---|---|---|---|---|---|
| Chlamydomonas reinhardtii | Cr2O3 | NR | 100 | NR | [36] |
| Chlorella vulgaris | Cu | 100 | 0.67–4 | 20 | [37] |
| Chlorella vulgaris | Se | 100 | 0.4-4 | 40–45 | [37] |
| Chlamydomonas reinhardtii | AgNP | 30–50 | 0.15 | NR | [39] |
| Scenedesmus obliquus | α-Fe2O3 | <30 | 0–20 | NR | [40] |
| Chlorella vulgaris | Y3Fe5O12 | <100 | 2.5 | 90 | [38] |
| Chlorella vulgaris | Fe3O4 | 50–100 | 10 | 90 | [38] |
| Chlorella pyrenoidosa | Fe2O3 | <30 | 20–30 | 33.75 | [29] |
| Scenedesmus | SiC | 25–100 | 150 | 23.53 | [41] |
| Nannochloropsis maritima | MNPs@Ag | 0.33 | 99.5 | [42] |
NPs: Nanoparticles
It was shown in the study that NPs such as Fe, Mg, Zn, Si, and Pb, among others, may be ideal for inducing biomass. Future biofuel production from algal biomass may benefit from an NP that causes greater lipid accumulation, but further research is necessary to fully grasp this potential benefit because there is little data and unambiguous evidence on the proper roles of size and concentration of various NPs.
4.3. Effect of NPs on Microalgal Lipid Production
Lipids are the main source of biofuel production [43]. Lipids are non-polar, insoluble in water and soluble in fatty solvents such as chloroform, benzene, methanol, etc. They can be further classified into polar (i.e., glycolipids, phospholipids) and neutral lipids (i.e., triglycerides). The biosynthetic pathways of lipids are completed in four steps, namely, carbohydrate accumulation in the cell; formation of acetyl CoA and Malonyl CoA; synthesis of palmitic acid and; synthesis of higher fatty acid by chain elongation. Two enzymes Acetyl-CoA carboxylase (ACCase) and fatty acid synthase are involved in the fatty acid synthesis [44].
Inoculation of NPs in the culture media can enhance their growth and lipid accumulation. It has been reported that ZnO-NP-treated Chlorella sp. showed a higher accumulation of triglyecerols [32]. The effect of iron oxide on microalgal growth and enhancement of biofuel were tested on species Chlorella pyrenoidosa and Chlorella sorokiniana [29]. Employing metal NPs to promote metal resistance in C. vulgaris leads to a boost in valuable products such as biomass, cellular pigments, and lipids derived from microalgae. Preliminary tests exhibited the development of metal resistance with metal NPs, and subsequent experiments validated their beneficial impact on microalgae growth, biomass production, and lipid synthesis in the presence of metal salts [47,57]. α-Fe2O3 showed up conflicting effects 20 mgL-1 dose of iron NPs enhanced the biomass concentration by 33.75% in C. Pyrenoidosa and at 30 mgL-1 dose, the highest lipid accumulation was shown (16.89 wt%), in contrast, at the lower dose iron NPs showed toxicity to C. sorokiniana. Similar studies were conducted on Scenedesmus obliquus and reported that α-Fe2O3 at 20 mg L-1 concentration enhanced lipid production by 39.6% [40]. Bio iron nano catalysts are also suggested to improve lipid production efficiency [48]. Low concentrations of the acquired NPs increased the biomass and lipid production of C. vulgaris, with 50 mgL-1 of ZnO NPs showing the greatest ability to increase [46]. According to the report, the presence of SiC and g-C3N4 NPs resulted in enhanced lipid accumulation, whereas the biomass recovery from TiO2 and TiC did not meet the desired level of performance. SiC NPs increased the absorption of light on Scenedesmus sp. under a xenon lamp, enhancing the lipid content by 32.07% [41]. Xenon lamp acts as solar simulators because they can create a wide illumination spectrum that is identical to that of solar radiation [45]. It is observed that eco-friendly NPs are cheaper, low on toxicity, and cost-effective [Table 2].
Table 2: Effects of NPs on lipid productivity.
| Microalgal species | NPs | Size | Concentration (mgL-1) | Lipid (%) | References |
|---|---|---|---|---|---|
| S. obliquus | Fe2O3 | <30 | 5 | 39.6 | [40] |
| S. obliquus | CNTs | <2 | 5 | 8.9 | [40] |
| S. obliquus | MgO | <50 | 40 | 18.5 | [40] |
| C. vulgaris | Mg | 82 | 150–200 | 393.33 | [47] |
| C. vulgaris | Cu | 89 | 10–20 | 86.67 | [47] |
| C. vulgaris | Zn | 92 | 150 | 333.33 | [47] |
| C. vulgaris | Pb | 76 | 50–100 | 206.67 | [47] |
| C. pyrenoidosa | Fe2O3 | <50 | 20–30 | 15.29 | [29] |
| Dictyococcus sp. VSKA18 | bio-iron (Sargassum polycystum) | 3.347 | NR | 44 | [48] |
| Coelastrella sp. M-60 | bio-iron (Sargassum polycystum) | 3.347 | NR | 52 | [48] |
| Scenedesmus sp. | SiC | 25-100 | 2900 | 32.07 | [41] |
| C. vulgaris | ZnO | 50 | NR | 59 | [46] |
S. obliquus: Scenedesmus obliquus, C. vulgaris: Chlorella vulgaris, C. pyrenoidosa: Chlorella pyrenoidosa, NPs: Nanoparticles
Thus, research has shown that the type and quantity of NPs utilized on the microalgae species have a significant impact on the lipid increase in microalgae. It is noteworthy that NPs, which have a beneficial impact on cellular activity, were used to accomplish the lipid rise.
4.4. Effect of NPs on Microalgal Toxicity
Over the past 10 years, nanoparticle applications have grown. A surge in applications indicated worry over their fate and actions in the environment. Particularly toward aquatic habitats, as aquatic bodies are where these NPs ultimately sink [49]. Due to their harmful effects on aquatic life, NP use has a significant negative influence on the environment. These NPs are more physiologically active than bigger-sized materials of the same chemistry because they have a larger surface area per mass. Assessing their ecotoxicological influence on aquatic ecosystems is crucial [50].
It is studied by Rana et al., that a lower dose of 2 mgL-1 of α-Fe2O3 IONPs caused toxicity to Chlorella sorokiniana. When Chlorella sp. were grown in conditions enriched with Cu, Pb, Mg, and Zn NPs, a comparable decline in their populations was also seen [51]. Chlorella vulgaris growth and biomass (dry weight, Chlorophyll A, and total chlorophyll) were drastically reduced by titanium oxide. Due to surface adsorption of the NPs on the algal cell, which promotes growth inhibition, TiO2 NPs can adsorb Zn and P from the algal growth media. The oxidative damage caused by TiO2 NPs to the algal cells was greatly worsened by the acidity [52]. The divalent form of Ni (II) is very high in the aquatic environment. The biotoxicity of nickel oxide NPs on Chlorella vulgaris was caused by their unavoidable effects on the development of aquatic photoautotrophs, cellular toxicity, morphological alteration, cell deterioration, and oxidative stress. These effects were related to the concentration of NPs and the production of ROS [53]. Silver NPs (Ag-NPs) are widely used in a variety of applications and have antimicrobial properties. Chlorella vulgaris, a marine microalga, was studied for Ag NP toxicity. Microalgae were exposed to different concentrations of Ag NPs (50 and 100 nm) for 96 h during exponential growth. Ag NPs negatively affected viable cell concentration, showed varied impacts on chlorophyll a, and increased ROS generation. Transmission electron microscopy revealed Ag NPs inside cells and forming large aggregates. Ag NPs disrupted carbon uptake, photosynthesis, and respiration, altering the growth kinetics and metabolism of C. vulgaris [54]. By causing cells to aggregate more readily, especially at higher doses, Ag NPs may have harmful effects on C. vulgaris [55]. The interaction between the protein-pigment complexes of the thylakoid membrane was thought to be affected by the Co NPs, which was hypothesized. It was notified by Chen et al., in marine microalgae Platymonas subcordiforus, Chaetoceros curvisetus and Skeletonema costatum that the release of Co2+ is the reason for CoNPs toxicity [56].
Cell structure, wall composition/thickness, membrane traits, and particle properties impact NP uptake, needing more research [30]. NP-cell interactions vary with NP qualities or membrane features. NPs are in commercial products, entering water systems. NP agglomeration affects aquatic ecosystems synergistically with pollutants like metals [31]. Algae exemplify ecosystems, demanding focused NP mechanism study due to their significance.
4.5. Effect of NPs on ROS
ROS are generated in algae when the equilibrium between the creation and suppression of ROS is disrupted by abiotic stressors, leading to an increase in ROS. In high quantities, ROS are extremely toxic and can cause oxidative damage [59]. Through excessive ROS generation, abiotic stressors produce oxidative stress. Biomolecules can interact with ROS and become inactive or altered, which can lead to organelle failure, changes in cellular structure, and mutagenesis [60]. Algae contain antioxidant defense mechanisms that counteract the negative effects of ROS and keep them alive during oxidative stress [61,62]. Zang et al., discovered that silver nanoclusters (AgNCs) and their released Ag+ affected the Calvin cycle and interrupted electron transfer in light reactions [63]. The toxic effect of Ag NPs was examined on two different habitat species Scenedesmus sp. (freshwater) and Thalassiosira sp. (marine diatom) by Pham [64]. The EC50 values of AgNPs, assessed after 72 h in Scenedesmus sp. and Thalassiosira sp which were 89.92 ± 9.68 and 107.21 ± 7.43 μg/L, respectively. After analyzing some points, such as half maximal effective concentration (EC50), algae growth inhibition, algae cell size, chlorophyll-a content, and total lipid accumulation, it has been concluded that Ag particles have different toxicity mechanisms for marine and freshwater. Compared to marine environments, freshwater has higher levels of Ag toxicity. It has been observed that results may vary from species to species. The microalgal cell wall is made up of lipids, polysaccharides, and glycoproteins, and depending on the type of metal ions and donor atoms in biomolecules, it can create various chemical interactions with semiconductor NPs [65-67]. It was observed in Chlamydomonas reinhardtii that the presence of 1000 mg/L CuO led to an increase in ROS concentration, which was coupled with lipid membrane peroxidation [68]. It was examined the toxicity of cadmium sulfide (CdS) and Zinc Sulphide (ZnS) NPs on Attheya ussuriensis, Chaetoceros muelleri, Porphyridium purpureum and Heterosigma akashiwo species [67]. A study reported that CdS NPs changed the biochemical composition, growth pattern, morphological pattern changes in the cell wall, and esterase activity in C. muelleri and P. purpureum while ZnS affected H. akashiwo and A. ussuriensis species. P. purpureum cell wall contains the phycoerythrin protein cell wall which is resistant to Zn2+ cations. The increased photoactivity of CdS NPs under visible light irradiation and lower dissociation in water, which enable them to produce more ROS and increase the risk of oxidative stress to aquatic animals, is the basis for their toxicity [69]. Iron NPs, via Fenton-type reactions, create diverse ROS that damage cells by oxidizing proteins and DNA thiol groups, causing lipid peroxidation and cell demise. ROS-induced toxicity, linked to cellular issues like membrane fluidity loss and lipid oxidation, must be controlled to avert cell death. NPs’ substantial surface area and electron density drive reactivity with biomolecules; their size significantly influences ROS formation.
4.6. Effect of NPs on Carbon Dioxide Sequestration
One of the biggest threats to humanity in the twenty-first century is the global risk posed by anthropogenic carbon dioxide (CO2) emissions. The growing interest in CO2 biodegradation as an environmentally acceptable method. Microalgae, known for adaptability, rapid growth, and cost-effectiveness, are globally recognized for this purpose. Through photosynthesis, they convert CO2 into biomass, usable for biofuels and valuable products. Enhanced cultivation techniques further boost microalgae’s ability to convert gases into biomass, elevating productivity [70]. The physiological state of the microalgal species, as well as environmental factors, including CO2 concentrations, pH, light intensity, and dissolved oxygen, can affect how effectively CO2 is captured by microalgae [71]. In the study, Graphene oxide quantum dots (GOQDs), excited by UV light (380 nm) and emitting blue light (465 nm), incorporated into Chlorella pyrenoidosa culture medium. It has been shown that the chlorophyll, in the GOQDs- combination efficiently received and used UV light, leading to considerably higher photosynthetic activity. In addition, the system showed a 34% increase in bioenergy accumulation and a 20% improvement in carbon dioxide fixing. In order to identify the biological response mechanism with GOQDs, further investigated the metabolic pathways of microalgae. Results showed that the GOQDs improved the photosynthesis of microalgae by facilitating photosystem II (PSII) energy transfer and upregulating the metabolites of lipid biosynthesis, leading to a larger biomass and lipid content [72].
Recent research on the physical adsorption technology using nanomaterials has produced encouraging findings in terms of boosting CO2 bio fixation by microalgae [71]. Among them, nanomaterials could significantly increase the amounts of ROS in microalgae and the rates of relative electron transfer in photosynthetic system II, hence enhancing the overall photosynthesis toward carotenoids.
4.7. Effects of NPs on the Environment
After NPs are released into the environment, they undergo various transformation processes that ultimately alter their destiny and interactions with other organisms [73]. Rapid commercialization is the main source of NPs release in the environment. NP, either individually or in conjunction with other contaminants like metals present in sediment and water phases, have the potential to form agglomerates and can collectively impact the structure, composition, and functioning of aquatic ecosystems through synergistic effects [74,75].
In agriculture, the use of NPs aims to enhance plant growth and development by facilitating the controlled release of agrochemicals. In the study, at the dosage of 1000 mg/kg, the utilization of ZnO NPs resulted in a notable enhancement in maize growth and grain yield compared to the untreated control [76]. On the other hand, the utilization of Astaxanthin NPs led to a substantial improvement in wheat plant growth, physiological functions, and nutritional attributes by alleviating the adverse effects of cadmium toxicity [77]. To achieve the commercialization of nano pesticides and nano fertilizers for global use, considerations of societal acceptance, economic viability, sustainability, and safety perspectives are crucial.
The higher concentration of NPs triggers the production of ROS, leading to damage in macromolecular structures such as nucleic acids, proteins, and lipids, as well as cellular organelles, ultimately reducing microbial cellular viability, causing toxicity to microbial communities including bacteria, fungi, protozoa, algae [78]. Research has demonstrated that titanium oxide NPs, when present at concentrations ranging from 1 to 100 mg/kg, did not exhibit toxicity to the soil microbial community. However, NPs of copper oxide, zinc oxide, and silver were found to be toxic to the soil microbial community at comparable concentrations [79]. Numerous in vitro and in vivo studies have provided evidence that NP exposure poses significant health hazards to humans, triggering inflammatory responses, myocardial infarction, oxidative stress, and thrombosis [80]. Cells internalize NPs through mechanisms such as macropinocytosis, endocytosis, phagocytosis, and passive penetration [81]. Prolonged exposure and accumulation of NPs within cells can lead to neurotoxicity through several mechanisms, including the buildup of autophagosomes, membrane damage, the formation of ROS, and interference with the cell cycle [82].
Understanding the fate and impacts of NPs in diverse contaminated environments is crucial for setting contamination prevention guidelines. In-depth in vivo studies are required for assessing the long-term effects, efficacy, reliability, fate, and toxicity of metallic NPs. Analytical and modeling advancements are needed to detect, estimate, and characterize NPs in environmental systems and consumer products. NPs’ multi-functional roles as micronutrients and protective agents in agriculture should be explored for improved sustainability. Life cycle assessments and economic analyses will further determine the viability of nanoparticle-enhanced biodiesel production. These efforts collectively pave the way for efficient, eco-friendly, and economically feasible microalgae-based biodiesel systems. Future research should optimize dosing and safe NP delivery for their potential in agriculture, environment, and medicine. Emphasis should shift to biocompatible, biodegradable NPs to curb environmental accumulation.
5. CONCLUSION
In summary, enhancing microalgal growth and intracellular chemical accumulation could involve introducing NPs into microalgal cells. Current research mainly examines nanoparticle toxicity in microalgae cultivation, but the focus should shift toward leveraging NPs to augment growth and yield for effective biorefinery practices. Recent studies show interest in incorporating NPs for microalgal biofuel production. Algae are vital to aquatic food webs, meaning pollutants affecting them can impact other organisms. Proper assessment of nanoparticle risks in aquatic environments requires distinguishing between internalization in algal cells and subsequent harm. While NPs might boost lipid accumulation for future algal biomass-based biofuel production, further research is needed to grasp this potential due to limited data on NP size and concentration effects.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. FUNDING
There is no funding to report.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All data generated and analyzed are included within this review article.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Jasmine M. US EPA Proposes Higher Biofuel Blending Mandates for Next Three Years. S&P Global Commodity Insights. Available from:https://www.spglobal.com/commodityinsights/en/market-insights/latest-news/agriculture/120122-us-epa-proposes-higher-biofuel-blending-mandates-for-next-three-years [Last accessed on 2023 Jun 15].
2. Prasad TN, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, et al. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 2012;35:905-27. [CrossRef]
3. Choi HJ. Effect of eggshells for the harvesting of microalgae species. Biotechnol Biotechnol Equip 2015;29:666-72. [CrossRef]
4. Uzoejinwa BB, He X, Wang S, Abomohra AE, Hu Y, Wang Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production:Recent progress and future directions elsewhere worldwide. Energy Convers Manag 2018;163:468-92. [CrossRef]
5. Bahadar A, Khan MB. Progress in energy from microalgae:A review. Renew Sustain Energy Rev 2013;27:128-48. [CrossRef]
6. Chauhan A, Jindal T. Microbiological Methods for Environment, Food and Pharmaceutical Analysis. Berlin:Springer Nature;2020. [CrossRef]
7. Khan MI, Shin JH, Kim JD. The promising future of microalgae:Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 2018;17:36. [CrossRef]
8. Hazeem L. Single and combined toxicity effects of zinc oxide nanoparticles:Uptake and accumulation in marine microalgae, toxicity mechanisms, and their fate in the marine environment. Water 2022;14:2669. [CrossRef]
9. Ranjan A, Arora J, Chauhan A, Kumari A, Rajput VD, Sushkova S, et al. Applications and implications of nanoparticles in food industries. In:Sustainable Plant Nutrition in a Changing World. Cham:Springer;2022. 223-43. [CrossRef]
10. Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials:History, sources, toxicity and regulations. Beilstein J Nanotechnol 2018;9:1050-74. [CrossRef]
11. Baig N, Kammakakam I, Falath W. Nanomaterials:A review of synthesis methods, properties, recent progress, and challenges. Mater Adv 2021;2:1821-71. [CrossRef]
12. Chauhan A, Basniwal RK, Gurnani M, Rath P, Ranjan A, Rajput VD, et al. Environmental emissions of nanoparticles. In:Sustainable Plant Nutrition in a Changing World. Cham:Springer;2022. 245-79. [CrossRef]
13. Deviram G, Mathimani T, Anto S, Ahamed TS, Ananth DA, Pugazhendhi A. Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J Clean Prod 2020;253:119770. [CrossRef]
14. Onyeaka H, Miri T, Obileke K, Hart A, Anumudu C, Al-Sharify ZT. Minimizing carbon footprint via microalgae as a biological capture. Carbon Capture Sci Technol 2021;1:100007. [CrossRef]
15. Halbus AF, Horozov TS, Paunov VN. Self-grafting copper oxide nanoparticles show a strong enhancement of their anti-algal and anti-yeast action. Nanoscale Adv 2019;1:2323-36. [CrossRef]
16. SanitàG, Carrese B, Lamberti A. Nanoparticle surface functionalization:How to improve biocompatibility and cellular internalization. Front Mol Biosci 2020;7:587012. [CrossRef]
17. Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Åberg C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc 2013;135:1438-44. [CrossRef]
18. Domozych DS, Ciancia M, Fangel JU, Mikkelsen MD, Ulvskov P, Willats WG. The cell walls of green algae:A journey through evolution and diversity. Front Plant Sci 2012;3:82. [CrossRef]
19. Wang D, Tan J, Zhu H, Mei Y, Liu X. Biomedical implants with charge-transfer monitoring and regulating abilities. Adv Sci (Weinh) 2021;8:e2004393. [CrossRef]
20. Cao M, Huang X, Wang F, Zhang Y, Zhou B, Chen H, et al. Transcriptomics and metabolomics revealed the biological response of Chlorella pyrenoidesa to single and repeated exposures of AgNPs at different concentrations. Environ Sci Technol 2021;55:15776-87. [CrossRef]
21. El-Naggar NE, Hussein MH, Shaaban-Dessuuki SA, Dalal SR. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci Rep 2020;10:3011. [CrossRef]
22. Laroche C. Exopolysaccharides from microalgae and Cyanobacteria:Diversity of strains, production strategies, and applications. Mar Drugs 2022;20:336. [CrossRef]
23. Babiak W, Krzeminska I. Extracellular polymeric substances (EPS) as microalgal bioproducts:A review of factors affecting EPS synthesis and application in flocculation processes. Energies 2021;14:4007. [CrossRef]
24. Sun J, Shi H, Mo T, Zhang Y, Wang X, Ding C, et al. Protein binding on the surface of magnetic nanoparticles. Part Part Syst Charact 2019;36:1900072. [CrossRef]
25. Wang S, Yerkebulan M, Abomohra AE, El-Khodary S, Wang Q. Microalgae harvest influences the energy recovery:A case study on chemical flocculation of Scenedesmus obliquus for biodiesel and crude bio-oil production. Bioresour Technol 2019;286:121371. [CrossRef]
26. Manzanares D, Ceña V. Endocytosis:The nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics 2020;12:371. [CrossRef]
27. Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, et al. Cellular uptake of nanoparticles:Journey inside the cell. Chem Soc Rev 2017;46:4218-44. [CrossRef]
28. Qiu S, Wu Z, Chen Z, Abbew AW, Li J, Ge S. Microalgal activity and nutrient uptake from wastewater enhanced by nanoscale zerovalent iron:Performance and molecular mechanism. Environ Sci Technol 2022;56:585-94. [CrossRef]
29. Rana MS, Bhushan S, Sudhakar DR, Prajapati SK. Effect of iron oxide nanoparticles on growth and biofuel potential of Chlorella spp. Algal Res 2020;49:101942. [CrossRef]
30. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2021;20:101-24. [CrossRef]
31. Donia DT, Carbone M. Fate of the nanoparticles in environmental cycles. Int J Environ Sci Technol 2018;16:583-600. [CrossRef]
32. Kaliamurthi S, Selvaraj G, Cakmak ZE, Korkmaz AD, Cakmak T. The relationship between Chlorella sp. and zinc oxide nanoparticles:Changes in biochemical, oxygen evolution, and lipid production ability. Process Biochem 2019;85:43-50. [CrossRef]
33. Huang W, Zhou Y, Zhao T, Tan L, Wang J. The effects of copper ions and copper nanomaterials on the output of amino acids from marine microalgae. Environ Sci Pollut Res Int 2022;29:9780-91. [CrossRef]
34. Pikula K, Chaika V, Zakharenko A, Markina Z, Vedyagin A, Kuznetsov V, et al. Comparison of the level and mechanisms of toxicity of carbon nanotubes, carbon nanofibers, and silicon nanotubes in bioassay with four marine microalgae. Nanomaterials (Basel) 2020;10:485. [CrossRef]
35. Munk M, Brandão HM, Yéprémian C, CoutéA, Ladeira LO, Raposo NR, et al. Effect of multi-walled carbon nanotubes on metabolism and morphology of filamentous green microalgae. Arch Environ Contam Toxicol 2017;73:649-58. [CrossRef]
36. Costa CH, Perreault F, Oukarroum A, Melegari SP, Popovic R, Matias WG. Effect of chromium oxide (III) nanoparticles on the production of reactive oxygen species and photosystem II activity in the green alga Chlamydomonas reinhardtii. Sci Total Environ 2016;565:951-60. [CrossRef]
37. Mykhaylenko NF, Zolotareva EK. The effect of copper and selenium nanocarboxylates on biomass accumulation and photosynthetic energy transduction efficiency of the green algae Chlorella Vulgaris. Nanoscale Res Lett 2017;12:147. [CrossRef]
38. Zhu L, Li Z, Hiltunen E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant:Effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol Biofuels 2018;11:183. [CrossRef]
39. Sendra M, Yeste MP, Gatica JM, Moreno-Garrido I, Blasco J. Direct and indirect effects of silver nanoparticles on freshwater and marine microalgae (Chlamydomonas reinhardtii and Phaeodactylum tricornutum). Chemosphere 2017;179:279-89. [CrossRef]
40. He M, Yan Y, Pei F, Wu M, Gebreluel T, Zou S, et al. Improvement on lipid production by Scenedesmus obliquus triggered by low dose exposure to nanoparticles. Sci Rep 2017;7:15526. [CrossRef]
41. Ren HY, Dai YQ, Kong F, Xing D, Zhao L, Ren NQ, et al. Enhanced microalgal growth and lipid accumulation by addition of different nanoparticles under xenon lamp illumination. Bioresource Technol 2020;297:122409. [CrossRef]
42. Fu Y, Hu F, Li H, Cui L, Qian G, Zhang D, et al. Application and mechanisms of microalgae harvesting by magnetic nanoparticles (MNPs). Sep Purif Technol 2021;265:118519. [CrossRef]
43. Aratboni HA, Rafiei N, Garcia-Granados R, Alemzadeh A, Morones-Ramírez JR. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb Cell Fact 2019;18:178. [CrossRef]
44. Bonnefond H, Moelants N, Talec A, Mayzaud P, Bernard O, Sciandra A. Coupling and uncoupling of triglyceride and beta-carotene production by Dunaliella salina under nitrogen limitation and starvation. Biotechnol Biofuels 2017;10:25. [CrossRef]
45. Mohsenpour SF, Hennige S, Willoughby N, Adeloye A, Gutierrez T. Integrating micro-algae into wastewater treatment:A review. Sci Total Environ 2021;752:142168. [CrossRef]
46. Nada HG, Ali HE, El-Behery RR, Shanab SM, Elshatoury EH. Nanoparticles biosynthesized by Bacillus cereus filtrate and gamma rays enhancing Chlorella vulgaris biomass and lipid production. J Clust Sci 2022;33:2055-68. [CrossRef]
47. Sibi G, Kumar DA, Gopal T, Harinath K, Banupriya S, Chaitra S. Metal nanoparticle triggered growth and lipid production in Chlorella vulgaris. Int J Sci Res Environ Sci Toxicol 2017;2:1-8.
48. Vignesh NS, Vimali E, Sangeetha R, Arumugam M, Ashokkumar B, Ganeshmoorthy I, et al. Sustainable biofuel from microalgae:Application of lignocellulosic wastes and bio-iron nanoparticle for biodiesel production. Fuel 2020;278:118326. [CrossRef]
49. Singh D, Gurjar BR. Nanotechnology for agricultural applications:Facts, issues, knowledge gaps, and challenges in environmental risk assessment. J Environ Manage 2022;322:116033. [CrossRef]
50. Saxena P, Sangela V, Harish. Toxicity evaluation of iron oxide nanoparticles and accumulation by microalgae Coelastrella terrestris. Environ Sci Pollut Res Int 2020;27:19650-60. [CrossRef]
51. Guldhe A, Kumari S, Ramanna L, Ramsundar P, Singh P, Rawat I, et al. Prospects, recent advancements and challenges of different wastewater streams for microalgal cultivation. J Environ Manage 2017;203:299-315. [CrossRef]
52. Roy B, Chandrasekaran H, Krishnan SP, Chandrasekaran N, Mukherjee A. UVA pre-irradiation to P25 titanium dioxide nanoparticles enhanced its toxicity towards freshwater algae Scenedesmus obliquus. Environ Sci Pollut Res Int 2018;25:16729-42. [CrossRef]
53. Adochite C, Andronic L. Aquatic toxicity of photocatalyst nanoparticles to green microalgae Chlorella vulgaris. Water 2020;13:77. [CrossRef]
54. Hazeem LJ, Kuku G, Dewailly E, Slomianny C, Barras A, Hamdi A, et al. Toxicity effect of silver nanoparticles on photosynthetic pigment content, growth, ROS production and ultrastructural changes of microalgae Chlorella vulgaris. Nanomaterials (Basel) 2019;9:914. [CrossRef]
55. Khoshnamvand M, Ashtiani S, Chen Y, Liu J. Impacts of organic matter on the toxicity of biosynthesized silver nanoparticles to green microalgae Chlorella vulgaris. Environ Res 2020;185:109433. [CrossRef]
56. Chen X, Zhang C, Tan L, Wang J. Toxicity of Co nanoparticles on three species of marine microalgae. Environ Pollut 2018;236:454-61. [CrossRef]
57. Fazelian N, Yousefzadi M, Movafeghi A. Algal response to metal oxide nanoparticles:Analysis of growth, protein content, and fatty acid composition. BioEnergy Res 2020;13:944-54. [CrossRef]
58. Hassanpour M, Tafreshi SA, Amiri O, Hamadanian M, Salavati-Niasari M. Toxic effects of Fe(2)WO(6) nanoparticles towards microalga Dunaliella salina:Sonochemical synthesis nanoparticles and investigate its impact on the growth. Chemosphere 2020;258:127348. [CrossRef]
59. Rezayian M, Niknam V, Ebrahimzadeh H. Oxidative damage and antioxidative system in algae. Toxicol Rep 2019;6:1309-13. [CrossRef]
60. Ranjbar S, Malcata FX. Challenges and prospects for sustainable microalga-based oil:A comprehensive review, with a focus on metabolic and genetic engineering. Fuel 2022;324:124567. [CrossRef]
61. Eskander S, Saleh HM. Heavy metal-induced oxidative stress and related cellular process. In:Cellular and Molecular Phytotoxicity of Heavy Metals. Cham:Springer Nature;2020. 99-123. [CrossRef]
62. VranováE, InzéD, Van Breusegem F. Signal transduction during oxidative stress. J Exp Bot 2002;53:1227-36. [CrossRef]
63. Zhang L, Goswami N, Xie J, Zhang B, He Y. Unraveling the molecular mechanism of photosynthetic toxicity of highly fluorescent silver nanoclusters to Scenedesmus obliquus. Sci Rep 2017;7:16432. [CrossRef]
64. Pham TL. Effect of silver nanoparticles on tropical freshwater and marine microalgae. J Chem 2019;2019:1-7. [CrossRef]
65. Neagu D, Papaioannou EI, Ramli WK, Miller DN, Murdoch BJ, Ménard H, et al. Demonstration of chemistry at a point through restructuring and catalytic activation at anchored nanoparticles. Nat Commun 2017;8:1855. [CrossRef]
66. Magalhães-Ghiotto GA, de Oliveira AM, Natal JP, Bergamasco R, Gomes RG. Green nanoparticles in water treatment:A review of research trends, applications, environmental aspects and large-scale production. Environ Nanotechnol Monit Manage 2021;16:100526. [CrossRef]
67. Pikula K, Mintcheva N, Kulinich SA, Zakharenko A, Markina Z, Chaika V, et al. Aquatic toxicity and mode of action of CdS and ZnS nanoparticles in four microalgae species. Environ Res 2020;186:109513. [CrossRef]
68. Melegari SP, Perreault F, Costa RH, Popovic R, Matias WG. Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat Toxicol 2013;142-3:431-40. [CrossRef]
69. Wu A, Tian C, Jiao Y, Yan Q, Yang G, Fu H. Sequential two-step hydrothermal growth of MoS2/CdS core-shell heterojunctions for efficient visible light-driven photocatalytic H2 evolution. Appl Catal B Environ 2017;203:955-63. [CrossRef]
70. Sun H, Zhao W, Mao X, Li Y, Wu T, Chen F. High-value biomass from microalgae production platforms:Strategies and progress based on carbon metabolism and energy conversion. Biotechnol Biofuels 2018;11:227. [CrossRef]
71. Li S, Li X, Ho SH. How to enhance carbon capture by evolution of microalgal photosynthesis?Sep Purif Technol 2022;291:120951. [CrossRef]
72. Yang L, Su Q, Si B, Zhang Y, Zhang Y, Yang H, et al. Enhancing bioenergy production with carbon capture of microalgae by ultraviolet spectrum conversion via graphene oxide quantum dots. Chem Eng J 2022;429:132230. [CrossRef]
73. Ding X, Pu Y, Tang M, Zhang T. Environmental and health effects of graphene-family nanomaterials:Potential release pathways, transformation, environmental fate and health risks. Nano Today 2022;42:101379. [CrossRef]
74. Abd-Elhakim YM, Hashem MM, Abo-El-Sooud K, Hassan BA, Elbohi KM, Al-Sagheer AA. Effects of co-exposure of nanoparticles and metals on different organisms:A review. Toxics 2021;9:284. [CrossRef]
75. Abbas Q, Yousaf B, Amina, Ali MU, Munir MA, El-Naggar A, et al. Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments:A review. Environ Int 2020;138:105646. [CrossRef]
76. Subbaiah LV, Prasad TN, Krishna TG, Sudhakar P, Reddy BR, Pradeep T. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J Agric Food Chem 2016;64:3778-88. [CrossRef]
77. Zeshan A, Abdullah M, Adil MF, Wei D, Noman M, Ahmed T, et al. Improvement of morpho-physiological, ultrastructural and nutritional profiles in wheat seedlings through astaxanthin nanoparticles alleviating the cadmium toxicity. J Hazard Mater 2022;424:126511. [CrossRef]
78. Hu J, Xianyu Y. When nano meets plants:A review on the interplay between nanoparticles and plants. Nano Today 2021;38:101143. [CrossRef]
79. Asadishad B, Chahal S, Akbari A, Cianciarelli V, Azodi M, Ghoshal S, et al. Amendment of agricultural soil with metal nanoparticles:Effects on soil enzyme activity and microbial community composition. Environ Sci Technol 2018;52:1908-18. [CrossRef]
80. Persiani E, Cecchettini A, Ceccherini E, Gisone I, Morales MA, Vozzi F. Microplastics:A matter of the heart (and vascular system). Biomedicines 2023;11:264. [CrossRef]
81. Jiang X, Wu Y, Gray P, Zheng J, Cao G, Zhang H, et al. Influence of gastrointestinal environment on free radical generation of silver nanoparticles and implications for their cytotoxicity. NanoImpact 2018;10:144-52. [CrossRef]
82. Li J, Song Y, Vogt RD, Liu Y, Luo J, Li T. Bioavailability and cytotoxicity of Cerium-(IV), Copper-(II), and Zinc oxide nanoparticles to human intestinal and liver cells through food. Sci Total Environ 2020;702:134700. [CrossRef]
83. Wang F, Guan W, Xu L, Ding Z, Ma H, Ma A, et al. Effects of nanoparticles on algae:Adsorption, distribution, ecotoxicity and fate. Appl Sci 2019;9:1534. [CrossRef]
84. Choi OK, Hendren Z, Kim GD, Dong D, Lee JW. Influence of activated sludge derived-extracellular polymeric substance (ASD-EPS) as bio-flocculation of microalgae for biofuel recovery. Algal Res 2020;45:101736. [CrossRef]
85. Mahata C, Dhar S, Ray S, Das D. Flocculation characteristics of anaerobic sludge driven-extracellular polymeric substance (EPS) extracted by different methods on microalgae harvesting for lipid utilization. Biochem Eng J 2021;167:107898. [CrossRef]
86. Kim JY, Kim KY, Kim SM, Choi YE. Use of rare earth element (REE)-contaminated acidic water as Euglena gracilis growth stimulator:A strategy for bioremediation and simultaneous increase in biodiesel productivity. Chem Eng J 2022;445:136814. [CrossRef]
87. Aghabeigi F, Nikkhah H, Zilouei H, Bazarganipour M. Immobilization of lipase on the graphene oxides magnetized with NiFe2O4 nanoparticles for biodiesel production from microalgae lipids. Process Biochem 2023;126:171-85. [CrossRef]