1. INTRODUCTION
Globally, land covers 29% of the earth’s surface, with glaciers accounting for 10% of the land, 19% is barren land, 32.7% of productive land is agricultural land, 27% forest land, 9.9% shrub land, 0.7% urban and built-up areas, and 0.7% freshwater [1]. Among productive terrestrial ecosystems, the forest plays key roles in biodiversity conservation, carbon sequestration, and support for rural livelihoods [2-5]. Forest ecosystems have been classified primarily based on climate into boreal, temperate, and tropical ecosystems, with highly correlated distributions with land used and soil characteristics, with poor nutrient soils assigned to forests and high fertility soils assigned to agricultural land and grasslands. Forest productivity has been declining as a result of accelerated human and livestock population growth, forest fires, and exploitation of forest resources [6,7]. Human activities have resulted in continuous soil erosion, causing essential nutrient deficiencies in forest soils [8,9]. Moreover, the population of the world is expected to reach more than 9 billion shortly by 2050. There will be a huge challenge ahead to feed this growing population, and our prime target would be to produce crops sustainably without hampering the ecosystem [10,11]. So for maximum production, farmers overused chemical fertilizers to provide the main plant nutrient elements, such as nitrogen, phosphorus, and potassium [12]. Overuse of chemical fertilizers increases soil acidity, decrease organic matter, humus, and beneficial organisms in the soil, stunt plant development, change the pH of the soil, feeds pests, and trigger emissions of greenhouse gases (GHG) [13]. Although the inefficiency in absorbing the nutrients causes the leaching of chemicals that are detrimental to the ecosystem, i.e., eutrophication, which means an increase in nutrient input to the level of overenrichment in surface waters, which results in an increase in primary production but will induce associated side effects. Hence, it threatens life in water and increases GHG emissions [14,15]. Globally, about 55% of GHG emissions are reported from agricultural land, and 6–11% of CH4 emissions are from anthropogenic sources [16,17]. Annually, the consumption of nitrogen fertilizer for growing rice has reached 7.66 million metric tons, and adding too much nitrogen fertilizer will reduce nitrogen use effectiveness and increase soil GHG emissions from paddy fields [18,19]. Furthermore, chemical plant protection measures such as insecticide and fungicides application can reduce the population of essential pollinators and beneficial microorganisms in the soil [20,21].
In addition, due to intensive cropping in agriculture, the use of chemical fertilizers increased, which have high nitrogen and phosphorus contents and leach from agricultural fields into rivers, causing fish mortality, oxygen depletion, an acceleration of aquatic plant growth, and, finally, a decrease in water quality for a sustainable ecosystem [22-24]. Increasing nutrients in the aquatic ecosystem cause different bacteria to flourish, which results in a visible film or scum on the water’s surface that helps with algae decomposition and subsequently drops oxygen levels, eventually creating a dead zone where the aquatic animal cannot exist [25]. Various diseases developed as a result of the accumulation of synthetic chemicals through the food chain in birds and animals, having a direct impact on their population density and a negative impact on the ecosystem [26-28]. To facilitate sustainable agriculture in the production of food and fiber as well as to preserve and develop forests, the scientific community from across the world is concentrating on the employment of beneficial microbes [29-32]. By enhancing the soil with beneficial elements like nitrogen, vitamins, proteins, and water-holding capacity, biofertilizers are useful instruments in the agriculture ecosystem that help to lessen the negative effects of chemical fertilizers [33]. Microbes that are soil and plant-associated carry out valuable biogeochemical cycles and organic matter degradation to maintain a healthy ecosystem [34].
Hence, microbial-based biofertilizers are highly beneficial for soil health and sustainable agricultural production [35-37]. Plant growth-promoting microorganisms (PGPM) are found in microbial-based biofertilizers, which are given to seeds or soil to benefit their hosts by reducing phytohormone production, boosting soil nutrient availability, enhancing plant nutrient uptake, and strengthening their resistance to diseases [38,39]. Furthermore, microbial-based biofertilizers can increase stress tolerance, prevent the adverse effects of salinity, fix nutrients in the root zone, control the plant pathogen biologically, and enhance the production of crops sustainably and economically [40-42]. As a result of microorganisms’ metabolic activity in biofertilizers, there is an increase in volatile organic compounds that affect plants and bacteria to control plant growth [43,44]. The necessary inoculum for the microorganism’s growth and multiplication can also be provided using biofertilizers continuously in a field for 3–4 years, which avoids the need for additional biofertilizer applications [45]. Despite the high number of patents on microbial inoculants, only a limited number have gone through commercial production for agricultural application in Asia (mainly China, and India), North America and Europe [46,47]. Moreover, responses of biofertilizers over different types of soil, crop, and environmental conditions, technological aspects of production, shelf life, and proper recommendation of biofertilizers uses are hindering the usage among farmers [48]. Therefore, biofertilizers have so far been unable to gain creditability in the commercial fertilizer market from the respective stakeholders (i.e., from farmers, producers and traders) [49]. This emphasizes the importance of education on the quality of formulations, their production and usage, cost effectiveness, quality indicators, and regulated requirements for the provision of legitimate items into the market and end user satisfaction. Researchers need to improve the technology to mitigate this inconstancy [50].
This review synthesizes knowledge of biofertilizer applications to provide organized insights about different microorganisms in terms of their efficacy for crop production. The application of this new review is to address the challenges in technology development and application, to improve biofertilizer production technology for sustainable crop production and forestry management, and to obtain food safety and security for future generations. The overall outline of the review is shown in [Figure 1].
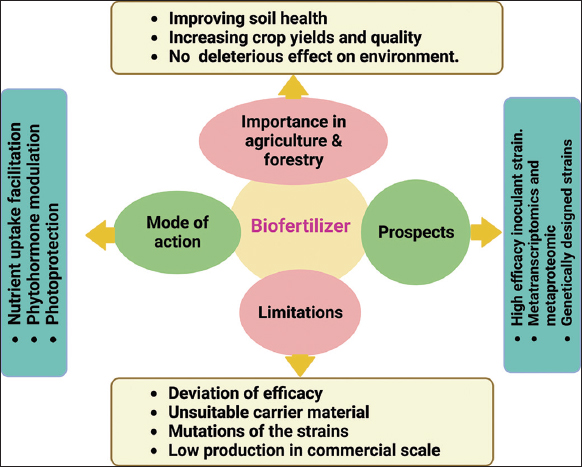 | Figure 1: Overview of the manuscript. [Click here to view] |
2. ADDRESSING BIOFERTILIZERS FOR SUSTAINABLE AGRICULTURE
Enhancing crop production for the growing people of the world is a significant task in the 21st century, while maintenance of ecological harmony is necessary. In this regard, sustainable agriculture is a global issue now [51]. The success of sustainable agricultural principles depends on more than just increasing crop yield; it also depends on maintaining environmental safety [52]. Chemical fertilizers should not be used in excess, since the residues have a negative impact on the soil’s ability to retain water and fertility and, most importantly, create an imbalance in the nutrients in the soil [53]. The human body will acquire hazardous compounds from plants grown with chemical fertilizers and pesticides [54]. Toxic chemicals and gases, including NH4, CO2, CH4, and others, are produced and released into the atmosphere as byproducts during the production of chemical fertilizers, marking the beginning of their negative impact on the environment [55]. Water pollution occurs when industrial waste is dumped into unfiltered water sources. Also covered is water eutrophication, the most damaging consequence of chemical waste accumulation in water bodies. Moreover, it is continued usage as a soil amendment leads to soil contamination by lowering soil quality [56,57].
In addition, only about half of the nitrogen fertilizer supplied to plants is actually used by them; the other 50% is lost to volatilization, organic compounds in clay soil reactions, and interference with surface and groundwater [58]. The most prevalent form of dissolved nitrogen found in groundwater or other bodies of water is nitrate, one of the crucial components of fertilizer. Human and animal health are negatively impacted by high concentrations of nitrites, nitrates, and nitrosamines [59]. High nitrate accumulation can result in (i) blue baby syndrome (acquired methemoglobinemia in infants); (ii) gastric cancer, (iii) other diseases such as goiter, congenital disabilities, and heart disease; and (iv) water eutrophication (mainly associated with nitrogen and phosphorus) [60].
The massive use of chemical fertilizers to increase crop productivity produces toxic GHGs that are degrading the ozone layer’s protective cover which means global warming and exposing people to dangerous ultraviolet rays [61]. Agricultural soils are the primary source of N2O emissions, accounting for 60% of anthropogenic N2O emissions [62]. The use of nitrogen fertilizer in excess contributes to the emission of nitrogen oxides (NO, N2O, and NO2), which is the main reason for air pollution [63]. The third most significant greenhouse gas after carbon dioxide and methane is nitrous oxide (N2O). It has 310 times more potential to cause global warming than carbon dioxide [64]. GHGs produced by the overuse of chemical fertilizers damage the climate. The regular use of chemical fertilizers exposes people to heavy metals like arsenic and cadmium, which are harmful to human health [65]. Using chemical fertilizers frequently could eventually lead to soil damage, the loss of microorganisms, and a host of other problems [66]. To make agriculture sustainable, it is necessary to implement a balanced and reasonable use of nutrients that are cost-effective and ecofriendly [67]. In this case, biofertilizer could be a suitable option [68,69].
The term “biological fertilizer,” also known as “micro inoculants” [37], was redirected to describe a substance containing living microorganisms that colonize the rhizosphere around the interior of the plant and promote development by improving the accessibility and uptake of mineral nutrients by the host plant [70,71]. Biofertilizers may solubilize plant nutrients and fix atmospheric nitrogen through the process of biological nitrogen fixation. They can also stimulate plant growth by synthesizing various growth-stimulating compounds, and their C: N ratio of 20:1 indicates their stability [72]. As biofertilizers are introduced, it is believed that the consumption of chemical fertilizers and pesticides will decrease [73]. In addition to allowing plants to obtain nutrients, some biofertilizers also produce several vitamins and phytohormones that contribute to plant growth [74,75]. Based on their purposes and modes of operation, biofertilizers are divided into many categories (Meena et al. [67]; [Table 1]). Agriculture techniques and the physical and chemical characteristics of the soil play a big role in the prevalence of bacteria in the soil. To achieve sustainable agriculture objectives in the future, nitrogen fixation and plant growth promotion by bacteria will be essential. Microbes also contribute to the ecosystem’s numerous nutrient cycles. According to the type of microbes utilized and their mode of action, biofertilizers are categorized in Table 1 with appropriate examples.
Table 1: Classification of biofertilizers with reaction mechanism and examples.
| Biofertilizers | Crops | Reaction mechanism | Groups | Examples | References |
|---|---|---|---|---|---|
| Nitrogen-fixing | Chickpea, white spruce | Increase soil nitrogen content by fixing atmospheric N and making it available to the plants | Free-living | Azotobacter, Anabaena, Clostridium, Aulosira, Bejerinkia, Nostoc, Klebsiella, Stigonema, Desulfovibrio, Rhodospirillum, and Rhodopseudomonas | [186-190] |
| Pea | Symbiotic | Rhizobium, Frankia, Anabaena azollae, and Trichodesmium | |||
| Lavendar | Associative symbiotic | Azospirillum spp., Herbaspirillum spp., Alcaligenes, Enterobacter, Azoarcus spp., and Acetobacter diazotrophicus | |||
| Phosphorus solubilizing | Chickpea, Wheat, Mangrove | Solubilize the insoluble forms of P in the soil into soluble forms by secreting organic acids and lowering soil pH to dissolve bound phosphates | Bacteria | Bacillus circulans, Bacillus subtilis, Pseudomonas striata, Penicillium spp., Bacillus polymyxa, Microccocus agrobacterium, Aereobacter and Flavobacterium | [187,191-194] |
| Wheat | Fungi | Penicillium spp., Aspergillus awamori, and Trichoderma | |||
| Phosphorus mobilizing | - | Transfer phosphorus from the soil to the root cortex | Mycorrhiza | Arbuscular mycorrhiza, Glomus spp., Gigaspora spp., Acaulospora spp., Scutellospora spp., and Sclerocystis spp. | [195] |
| Potassium solubilizing | - | Solubilize potassium (silicates) by producing organic acids that decompose silicates and help in the removal of metal ions and make them available to plants | Bacteria | Bacillus mucilaginous, Bacillus circulanscan, Bacillus edaphicus, and Arthrobacter spp. | [196] |
| - | Fungi | Aspergillus niger | |||
| Potassium mobilizing | Wheat | Mobilize the inaccessible forms of potassium in the soil | Bacteria | Bacillus spp. | [197-199] |
| Maize | Fungi | Aspergillus niger | |||
| Micronutrient | Onion, Maize, Pumpkin | Oxidizing sulfur to sulfates that are usable by plants | Sulfur oxidizing | Thiobacillus spp. | [74,200,201] |
| Wheat, Maize | Solubilize the zinc by proton, chelated ligands, acidification, and by oxidoreductive systems | Zinc solubilizing | Mycorhiza pseudomonas spp., and Bacillus spp. | [202-204] | |
| Plant Growth Promoting | Rice, Bitter gourd, Gladiolus, Chrysanthemum, Petunia, Pinus | Produce hormones that promote root growth, improve nutrient availability, and improve crop yield | Plant growth-promoting rhizobacteria | Pseudomonas spp., Agrobacterium, Pseudomonas fluorescens, Arthrobacter, Erwinia, Bacillus, Rhizobium, Enterobacter, Streptomyces, and Xanthomonas | [83,205-210] |
The microbial community modifies the soil by improving nutrient availability, nutrient uptake, and nutrient solubility [76]. Bacteria play an important role in the availability of essential micronutrients and macronutrients to plants. The main jobs of microorganisms include fixing nitrogen in the soil, converting phosphorus into a form that plants can absorb, synthesizing compounds that stimulate plant development, and protecting plants from pathogenic organisms that can infect them with illness [77,78]. In addition, bacteria create phytohormones, which play a significant role in controlling plant growth, have a significant impact on plant metabolism, and promote the plant’s reaction to stress [79].
3. BIOFERTILIZER APPLICATION IN AGRICULTURE
The use of biofertilizers in the agriculture sector has become increasingly popular due to thorough studies on agricultural crops, but there is comparatively limited documentation throughout the literature regarding their effects on forestry species [80].
3.1. Mode of Action of Biofertilizer
The different mechanisms of action of biofertilizers, including nutrient uptake facilitation, phytohormone regulation, and phytoprotection, must be understood to effectively utilize their potential for increasing the ecological services of forest biomes and promoting production in agriculture sectors [32]. By supplying vital nutrients such as nitrogen, phosphorous, and potassium, biofertilizers preserve the soil’s natural qualities (Aloo et al. [81]; [Figure 2]). PGPMs are categorized into three major divisions: Arbuscular mycorrhizal fungi (AMF), plant growth-promoting rhizobacteria (PGPR), and nitrogen-fixing rhizobia [82]. PGPRs help plants grow and develop by making it easier for plants to take in nutrients (N2 fixation and P solubilization), causing the root surface to grow (hormone production), or reducing the damage caused by pathogens, which is a sustainable way to increase crop yield [83]. Beneficial bacteria, including RHIZOBIUM [84], AZOTOBACTER [85], and AZOSPIRILLUM [86], have the capacity to fix atmospheric nitrogen into a form that is easily available for plant absorption and support plant development [Figure 3]. Phosphorus-solubilizing microorganisms, including PSEUDOMONAS and BACILLUS, may solubilize and mineralize both organic and inorganic phosphorus [87], making it available for plant absorption. In addition, several biofertilizers, such as AZOTOBACTER and AZOSPIRILLUM, release chemicals that promote root growth and aid in enhancing nutrient uptake [88,89]. Then, some biofertilizers, including AZOTOBACTER, AZOSPIRILLUM, and BACILLUS [90], release hormones that encourage plant growth, including auxins [91], gibberellins, and cytokinins. In addition to supporting root growth, blooming, and fruiting, these hormones assist in controlling plant growth and development.
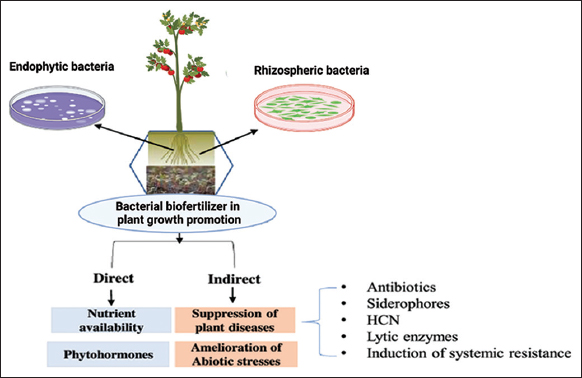 | Figure 2: The beneficial mechanisms of microbial strains as a biofertilizer and their role in maintaining soil fertility and enhancing crop productivity. [Click here to view] |
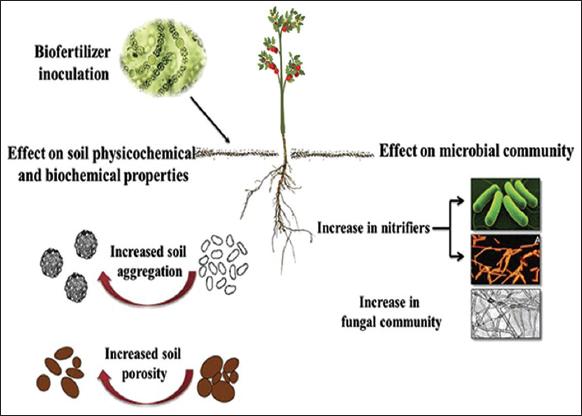 | Figure 3: Role of biofertilizers on physiological and biochemical properties of soil. [Click here to view] |
In addition, biofertilizers can shield plants from a variety of biotic and abiotic stressors [92,93]. By producing antibiotics and other antifungal substances, for instance, they help reduce the severity of soil-borne infections [94]. They can also improve soil fertility and reduce environmental pollution by binding metals, which decrease the toxicity of heavy metals [95]. In addition, biofertilizers can encourage the formation of phenolic compounds, which are naturally occurring poisons that help protect plants from harmful organisms, which in turn can promote plant defense mechanisms[96].
3.2. Status of Biofertilizer Application in Agriculture and Forestry
In many parts of the world, particularly in developing countries, market momentum has increased due to concerns about sustainable agricultural and forest management, notably integrated nutrient management. As a result, the use of biofertilizers AMF, phosphorus-solubilizing bacteria (PSB), AZOSPIRILLUM, AZOTOBACTER, RHIZOBIUM, ACETOBACTER, seaweeds, etc.) has received more attention [97,98]. Demand for biofertilizers has unexpectedly increased in China, Canada, Argentina, and Europe, particularly in Spain, Italy, and Germany, as well as in the United States, India, and Africa [99,100]. These nations are putting a lot of effort into promoting the use and development of biofertilizers as a result of their growing demand for organic products and the realization of the great benefits that these fertilizers provide [101-103]. The global organic agricultural area is increased from 69.4 million hectares in 2017–74.9 million hectares in 2020, according to FiBL data [104]. The consumption of organic products, including biofertilizers, has increased along with the adoption of more organic farming techniques, which has enhanced soil fertility. However, plants get benefits when biofertilizers are used as seed or soil inoculants because they grow and take part in the nutrient cycle. In agriculture and forestry, biofertilizers are becoming more popular since they offer an alternative to chemical fertilizers, are less expensive, improve soil health, and increase production by 10–25% without harming the soil or the environment [105]. The use of biofertilizers can also help to reduce soil erosion and improve water retention [106], making it an attractive option for agriculture and forestry. Recently, research has focused on biofertilizers to improve soil fertility by reducing the salinization of soil [107], phytoremediation of metal-contaminated soils [108], and increasing the tolerance capacity to extreme events [109] to maximize agricultural yields. Some of the research has progressed from the laboratory to the field trial stage. Research has focused on the continuous improvement of technology to reduce costs, maintain quality, and increase the popularity of biofertilizers among farmers. New technology such as nanotechnology, introduced as nano-fertilizers, nano-biofertilizers, nano-pesticides, and nutrients, has aided in the development of advanced, low-cost, and environmentally friendly fertilizers [82]. To restore functional and resilient agricultural microbiomes in the modern era, high-throughput sequencing has been proposed as an adequate strategy to select the “missing pieces” (i.e., those microbes involved directly and/or indirectly in soil P cycling whose populations were disturbed or unbalanced due to unsustainable intensive agricultural practices) [110,111].
3.3. Application of Biofertilizer in Forestry and its Impacts
In forest ecosystems, bio-fertilizers can be an integral part of integrated nutrient management where nitrogen fixers, potassium and phosphorus solubilizers, growth-promoting rhizobacteria (PGPRs), endo- and ectomycorrhizal fungi (ECMF), cyanobacteria, and other beneficial microorganisms are used to improve nutrient and water uptake, plant growth, and plant tolerance to abiotic and biotic factors [9]. ECMF play a crucial role in the nutrient cycle in terrestrial ecosystems, particularly in forest ecosystems where ECMF forms a symbiotic relationship by forming a mantle and Hartig network of intercellular hyphae in the roots of forest species [112]. This relationship provides significant benefits for the restoration of forests and ecosystem soil aggregation and stabilization [113]. In addition, ECMF and mycorrhizal helper bacteria promote the growth of economically significant trees and cut fertilizer costs in an ecofriendly manner [114].
More than 7000 different species of fungi generate ectomycorrhizae [115], many of which are associated with significant commercial trees such as poplar, birch, oak, pine, and spruce [116]. ECMFs are symbionts with most temperate and boreal forest trees, providing soil nutrients and water in exchange for plant carbon, and they are also used to restore sites contaminated with heavy metals, affected by soil erosion, and degraded due to clear-cut logging and wildfire [117]. ECMF nursery inoculation enhanced the performance of conifers planted in oil sands reclamation regions on degraded gypsum soil [118]. Even ECMF appears to be particularly efficient in recovering heavy metal-contaminated forest soils [119]. Recent years have seen the discovery of many ECMF isolates and associated hosts with increased metal tolerance [120]. In tropical forests of New Caledonia that have been degraded by mining activity, the ECMF species Pisolithus albus has been employed as an inoculant of ectomycorrhizal endemic hosts (Acacia spirorbis and Eucalyptus globulus) to enhance plant growth and act as a protective barrier to hazardous metals [121].
In addition, ECMFs were used to restore clear-cut logging sites, and it was found that soil transfers from mature adjacent forests helped form ectomycorrhizas by getting rid of harmful rhizosphere bacteria. This makes the clear-cut habitat a good place for organisms that were already living there [122]. Moreover, depending on the intensity and frequency of fire, some ECMF species have the ability to sustain forest fires; these fungi may be important for bringing in new ectomycorrhizal plant hosts and making the soil more stable through leached nitrogen (ammonium (NH4+) and nitrate (NO3- form) captured and transferred to remaining hosts in the post-fire area [123,124]. ECMF also has potential for use in restoring sites invaded by non-native plant species [125]. The invasion of pine trees into the Southern Hemisphere is one of the most widespread invasions of non-native species on Earth. It has turned native forests, grasslands, and shrublands into forests dominated by conifers and forced different ECMF communities to live together in their natural habitats [126]. However, pine invasions into native forests are always linked to a small number of non-native and invasive ECMF species taking over the roots of the pine [127]. Even though Pinaceae species spread quickly, they are often planted for forestry [128]. A different way to stop or slow the spread of pine hosts is to use non-invasive ECMF as inoculants for new plantations.
Plant growth is also affected by the make-up and activity of the associated bacterial community (phytomicrobiome), especially in the rhizosphere [129]. Plant-growth-promoting rhizobacteria (PGPR) can stimulate many direct and indirect mechanisms, such as phosphate solubilization, nitrogen fixation (N2-fixing), and phytohormone production [130]. Early seedling establishment is difficult in semi-arid forests, particularly in oak (Q. brantii) forests in Western Iran, but the use of biochar and PGPR is effective in controlling water stress in oak seedlings [131]. Brevibacillus reuszeri MPT17 strain of PGPR helps Carya illinoinensis roots grow and boosts nutrient levels in plants and the soil around their roots, especially by making more phosphorus and potassium available [132]. Rhizosphere-promoting bacteria, such as Bacillus paramycoides JYZ-SD5, and the ectomycorrhizal fungus, Schizophyllum commune, both help Metasequoia glyptostroboides grow even when it is under a lot of stress from salt [133]. Furthermore, nitrogen (N2-) fixation by moss-associated CYANOBACTERIA can promote moss growth and is an important source of new nitrogen in Eastern Canadian boreal forest ecosystems [134]. CYANOBACTERIA conduct massive amounts of nitrogen to forest ecosystems through nitrogen fixation, colonize feather mosses such as Hylocomium splendens and Pleurozium schreberi in the subalpine forests of Mt. Fuji [135].
Studies have shown that the use of biofertilizers in silviculture can increase tree growth, yield, and quality [136]. The AZOTOBACTER was found to be more effective at increasing organic matter and nitrogen, whereas the PSB treatment is more effective at increasing phosphorus and potassium. The PSB mobilizes restricted Phosphorus into a form that is available to the plants, which leads to the growth of seedlings [80]. As some examples, growth promoting bacteria isolates such as Lysinibacillus sphaericus, Paenibacillusquercus, Paramyrothecium roridum, and Lysinibacillus fusiformis displayed significant potentiality to increased growth of Eucalyptus pellita. The application of this biofertilizer to developing or recently grown Eucalyptus pellita in the field warrants further study. To determine whether the isolated strains from the consortia are successful at promoting plant growth in the cultivation of Eucalyptus pellita under pot trial conditions [137]. Forest biomes are rich in microbes and many aspects of the dynamics of microorganism values in their assemblages and their interactions with hosts remain unknown and require further investigation.
4. CHALLENGES OF BIOFERTILIZER APPLICATION
Biofertilizers are renewable, ecofriendly, safe, and cost-effective; however, they face some application-related difficulties. It has sluggish action, formulation complexity, high sensitivity to temperature and moisture, absence of a specific microbial strain, lack of suitable facilities for the manufacture of biofertilizer, lack of skilled labor, seasonal-based demand due to microbial activity, production regulation, market demand, and other issues that affect the impact of this approach in production, marketing, or practicality and usage [138]. However, as demonstrated by the use of AZOSPIRILLUM, the effectiveness of any biofertilizer application in forestry depends on the creation of microbial strains and inoculums, including formulations and field testing methodologies [139].
4.1. Formulation Complexity
The use of biofertilizers is receiving more attention and several of the products are currently offered globally [140]. In biofertilizers with living microbial cells, the formulation is one of the key elements that greatly influence the quality of the biological agents [35]. The preparation of inoculants is a critical multi-step procedure that involves one or more strains of microorganisms, an appropriate carrier, and additives that offer a safe habitat to protect them in challenging circumstances during storage, survival of strains, transportation, and establishment after introduction into soils. An efficient preface of microorganisms at the target region is provided by the right formulation, which also increases their activity once they have been injected into the host [141,142]. Another meaningful aspect is cost-effectiveness, which should be considered when selecting formulated products [143]. For commercial biofertilizer production, different carriers and organic matter should be available and cheap [144].
However, one of the key challenges for developing an improved formulation under field conditions is that a microorganism shows promising results under laboratory conditions. Manufacturers chose two or more types of microorganisms (e.g., RHIZOBIA and AMF, RHIZOBIA and PSB, diverse strains of AMF, or PSB) in a single product which help to maximize the resulting benefits for the host plants shown in several studies [39,71]. There are four types of biofertilizers, depending on the carrier’s physical characteristics and the material employed: formulations using solid carriers, liquid carriers, formulations with polymer entrapped carriers, and pressurized dry carriers [145].
Beneficial microorganisms’ metabolic activity quickly declines after production in liquid formulations [15,146,147]. Contrarily, solid formulations are problematic for non-sporulating bacteria because desiccation damages cell membranes, leading to cell death and loss of viability during rehydration, which has a significant negative impact on the commercialization of the product [148]. Despite the benefits of using the immobilized-cell formulation, it has still a limitation in large-scale production and field application because of the relatively high production cost [49,71,149,150], for the reason that the cost of polymeric carrier is high than the other methods [151]. Furthermore, cell mortality is one of the key difficulties of the bio-encapsulation process during the drying of encapsulated cells [150]. One of the major limits for polymer entrapped formulation is the survival of inoculums as low oxygen transfer [152].
Short shelf life and contamination are the key drawbacks of fluidized-bed dry formulation because of the moisture content of carrier-based inoculants [153]. In addition, safeguarding the finished product against infection is essential because unauthorized microorganisms have the power to completely alter the strain’s characteristics. However, it is essential to generate the strain in a sterile setting, which could raise production costs [154]. However, it is needed to improve formulations which are more effective, stable over time, better in quality, more consistent, cost-effective, and meet farmers’ needs.
4.2. Efficacy Changes with Climatic Conditions
The harsh and unpredictable environmental condition affects the efficacy of bio-formulation technology. Inoculants are subjected to biotic stress factors (such as microflora and microfauna) as well as abiotic stress conditions (such as soil pH, temperature, and salinity) during soil application [148]. In semi-arid environments, introduced inoculums struggle to survive inclement weather, including droughts, high salinity, inadequate irrigation, and even soil erosion, which quickly depletes the imported bacteria. In addition, bacteria have poorer tolerance for physical stress, particularly different temperature variations that occur during storage in peat carriers [152]. Due to their sensitivity to diverse environmental circumstances such as heat or drought, several biocontrol strains of bacteria are Gram-negative, which complicates their bioformulations [155]. Therefore, changing temperatures affect how adaptable and useful the microbial strains are: To solubilize Phosphorus (P) in pine forests under subfreezing temperatures, a PSB that is adapted to cold climates should be physiologically active. As a result of their functionality at room temperature, however, the psychrotolerant PSB species are likewise excellent options for P biofertilizers in any situation [156]. Nevertheless, the frequent use of plant growth promoting bacteria (PGPB) faces challenges during applications due to its inconsistent behavior and viability, particularly because PGPB cannot efficiently cover many plant species, its field application can generate inconsistent productivity, and the surrounding environment and microbial community can affect PGPB activity [83,157]. Finally, because of their poor growth in the culture system compared to their native habitat, many isolated PGPB species may find massive production difficult [158].
However, including giving due consideration to the rapidly changing global climate and region-specific forecasted conditions over the coming decades, which may affect product suitability and efficacy.
4.3. Storage Conditions
Microbial survival, carrier material qualities, biological effectiveness, and product storage life are all affected by storage conditions such as temperature, humidity, and sunlight intensity [159]. The carrier should maintain the viability of the microorganisms during storage in the farmer’s warehouse and have a lengthy shelf life and stability [150]. For the biofertilizer to be effective in the field, it must contain a sufficient number of viable cells during storage [160]. The shelf life of biofertilizers is another significant problem that can occur while using them. Live microbial cells used in biofertilizers have a limited shelf life (about 4–6 months under 20–25°C), and their storage and shipment require additional care and attention, increasing the cost of the product [110]. Biofertilizer should be stored properly, particularly at the correct temperature, to prevent the number of viable bacterial cells in the biofertilizer from falling below 108 cfu/mL. The lengthy shelf life of Acinetobacter baumannii, exceeding the required minimum of 108 cfu/mL at 6 months of storage, makes it a desirable biofertilizer product [161].
Biofertilizer should be kept cool, ideally in the refrigerator, to maintain their quality and shelf life. The recommended storage temperature for optimal efficiency and extended shelf life has been identified as 4°C [99]. The best storage temperature for Azotobacter venelandi NDD-CK-1 for up to 90 days was found to be 5°C, implying that inoculum can be stored at a low temperature for a prolonged shelf life [99]. On the other hand, Burkholderia spp. held at 28°C for 2 months grew more viable than those stored at 4°C, indicating that 28 °C is the best temperature for the studied rhizobia [160]. Whenever a 4°C inoculum is to be utilized, it must be cultured at 26°C for at least 7 days to commence microbial multiplication and achieve the requisite viable cell quantity. Biofertilizer stability and quality are very much dependent on storage conditions [162]. However, the above circumstance accounts for the product’s infrequent availability in rural locations [163].
4.4. Practicalities of Biofertilizer Application
Several methods of applying biofertilizers to soil exist, including root dipping, seed inoculation, and soil treatment using dry or liquid biofertilizers [29]. To create slurry for seed inoculation, carrier biofertilizers are diluted in water. To ensure that all of the inoculants are evenly distributed, sterile seeds are added to the slurry. The mixture is then air-dried before being planted. The root-dipping biofertilizer application technique is utilized for transplanted crops. Before being transplanted, the plant’s root is briefly submerged in a mixture of biofertilizer and water. Biofertilizer is applied as a foliar spray or as a soil application at a specific time when the farmer is prepared to plant the seed [164].
The use of biofertilizers is a biological strategy for the long-term sustainability of agriculture. However, there are significant obstacles to their use in enhancing agricultural output. As a result of biotic and abiotic stress, biofertilizers frequently perform less effectively in the field than they do in the laboratory or greenhouse [165]. Crops are grown in a variety of climates, soil types, levels of rainfall, crop varieties, and soil biodiversity. Because of these differences, the effectiveness of biofertilizers varies. In addition, because the inoculum needs time to colonize the root and increase its concentration, biofertilizers take longer to act than synthetic fertilizers [163]. The adoption of biofertilizers by farmers may be impacted by these reactions. The main issues with the use of biofertilizer include a lack of understanding within farmer communities of the value of microbial biofertilizer in terms of protecting the environment, inadequate encouragement, and promotion of the use of biofertilizer products by agricultural extension workers to farmers, a lack of acceptable carriers for the formulation of biofertilizer, and a lack of storage facilities [157,166]. In addition, because most biofertilizers are selective in their operations, the lack of labeling that indicates the expiration date and the identity of the microorganisms responsible for the biofertilizer’s formation may cast doubt on the validity of the materials [47].
4.5. Market Value
At present, people are focused on organic farming due to rising health concerns. As a result, consumers in developing countries are interested in biofertilizers, and the market for these products is expanding [167], as well as in developed countries such as Spain, Italy, and Germany, where there has been a surprising increase in biofertilizer demand [168]. The value of the worldwide biofertilizer market was $1.88 billion in 2022, and it is anticipated to reach $2.14 billion in 2023. With a compound annual growth rate (CAGR) of 13.2%, the biofertilizer market is projected to reach $3.51 billion in 2027 [169]. Legumes and N2-fixing inoculants now dominate the global biofertilizer market [170]. According to the literature, rhizobia-based inoculants account for about 78% of the global biofertilizer market, whereas phosphate solubilizes and other bio inoculants account for 15% and 7%, respectively [167,170].
As per reports, India is the world’s fourth-largest user of Potassium bio inoculants, whereas the United States, China, and Brazil top the list in terms of the total consumption of these microbial products [171]. When looking at the market by geography, specifically by continents, North America dominates the global market for biofertilizers, closely followed by Europe in second place and Asia-Pacific in third place [172]. The remainder of the world has been grouped, with South America rising to take the top spot. The biofertilizer industry is expanding internationally as a result of the desire to boost food production sustainably [70].
5. FUTURE RESEARCH DIRECTIONS
The importance of biofertilizers has been realized all over the world but research gaps still exist. The field efficiency of biofertilizers has to be improved by research and development. It may be possible to identify a unique application and enhance the inherent potential of the soil and the plant microbiome with new insights into how beneficial bacteria encourage plant growth. Plant development and growth are significantly influenced by the microbial ecosystem that already exists in plants. As a result, researchers need to focus on the biofertilizers’ capacity to alter the pre-existing microbiome [99]. In addition, the topic of forest biofertilizers has a significant research gap. For deciduous trees, no field data have ever been gathered yet, but coniferous species have collected few field data. Further, the majority of laboratory and greenhouse experiments have failed to transfer to the field. Hence, future research in this area could provide valuable information that will help us to comprehend the many biotic interactions that take place in any forest [32].
It is important to discover and develop inoculant strains of high efficiency, which should be effective under different soil conditions and plant species. The study of mechanisms related to the ecology and physiology of RHIZOBACTERIA as biofertilizers in different soil conditions has to be investigated elaborately. Therefore, it is necessary to conduct an in vitro and in vivo investigation of the physiology of inoculant cells in various soil types. In addition, careful research must be done on how bacteria are monitored after inoculation and how they evolve over time. Therefore, it is necessary to develop cutting-edge molecular analysis, visualization technology, microorganism engineering, biotechnology, and functional genomics investigations [173].
Metagenomics has a role in better understanding microbial communities, and it is a growing field. In the case of a plant rhizosphere, it is a colony of different microorganisms, and these microbial genes interact with plant genes [174]. However, it is important to conduct more elaborate research on areas such as metatranscriptomic and metaproteomics and their relation with plant growth [175]. Initial biofertilizers are hosts of non-transformed bacterial strains, which results in good efficiency. However, the establishment of genetically modified strains is necessary because they are becoming more and more successful at stimulating plant development. There is a wrong perception among general people that genetically designed strains are not safe for the environment [93]. Researchers and communicators have to demonstrate to general people and administrations that these strains do not pose any hazards. These biofertilizers must have the potential to support microbial life in unfavorable soil environments. Furthermore, financial evaluations for various agricultural outputs should be conducted [176].
According to keystone taxa, biofertilization can be employed to maximize the results by controlling the growth and function of other individuals in the plant microbiome. Designing microbial communities can benefit from greater research into these taxa. In addition, novel isolates exhibiting biofertilizer features should be found using culture-based methods [177]. The efficiency of biofertilizers can be increased by plant prebiotics, which function as signaling molecules to draw in helpful bacteria. It is important to keep track of the findings from these investigations conducted under various environmental circumstances, targeted plant genotypes, soil types, and growing seasons in a global database [110].
Azospirillum contributes to plant growth enhancement through N-fixing, and later research revealed that glutamine synthetase mutants of Azospirillum provide greater growth than the original type [86]. Similarly to this, various mutants can be produced, examined, and compared with their parental types to produce better results in the future. Plant growth and development are directly impacted by several enzymes, including 1-aminocyclopropane-1-carboxylate (ACC). The ACC deaminase gene was discovered, extracted from Pseudomonas putida, and introduced into other bacteria to create PGPB [89], which in turn stimulates plant growth and productivity. Therefore, we should look for these genes and use biotechnology techniques to create genetically engineered plant growth-promoting bacteria in the future, which offer a new strategy for indestructible agricultural growth.
A lot of research has shown that some strains of the three main groups of microorganisms in the rhizosphere — AMF, yeasts, and bacteria — can make their host plants more resistant to drought by showing different plant growth promoting (PGP) traits. With this information in mind, it is possible to think that using different PGP microorganisms at the same time could help their host plants, as long as their coinoculation does not lead to reactions that are harmful to each other. Using single Omics methods such as genomes, metabolomics, or proteomics, such effects have only partially been studied to date [178-181]. The application of the biofertilizer combined with reduced synthetic N fertilization could maintain the yield, reduce the input of synthetic fertilizer, and improve economic efficiency [182,183]. Reducing the amount of chemical fertilizer used and adding JUNCAO nitrogen-fixing biofertilizer (a 75% rate of chemical fertilizer and JUNCAO nitrogen-fixing biofertilizer) showed signs of improved crop vigor in P. giganteum. It also improved the nutritional quality of the herbage and the soil nutrient status of P. giganteum to a moderate degree, and it cut the cost of fertilizer [184]. In addition, farmers have high expectations for the subsidy policy of biofertilizer application in the future agricultural policy, and the subsidy policy is an effective incentive to promote the use by farmers [185]. Hence, we can modulate their combined use and drive it to increase crop yields, improve production processes, and meet rising global food demand using multi-omics approaches to understand in depth the processes that occur in plants when microorganisms are present.
6. CONCLUSION
Biofertilizers are a necessary component of environmentally friendly agricultural operations since it is important to reduce the impact on the environment. Despite the lack of data, it is obvious that biofertilizers have a significant role in agricultural productivity and also show great potential for the restoration of forests and the environment. An effective biofertilizer can have a good impact on plant development activities when it is correctly matched with the species of the host plant. Therefore, with a better understanding of the metagenomics of microorganisms, it will be possible to develop a proper environmentally-friendly fertilizer to be commercially used in agriculture. For the creation of efficient biofertilizers for sustainable agriculture and forestry, problems with host compatibility, storage conditions, formulation complexity, and other factors must be resolved. The development of efficient, innovative biofertilizer delivery systems that can be used to enhance in situ reforestation efforts using biofertilizers is a key area for future research. Therefore, biofertilizers are one of the key answers, we must take into account if we are to establish a sustainable farming system that will feed the entire world while making a profit and improving both human and environmental well-being.
7. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
8. FUNDING
This study was supported by the Heilongjiang Touyan Innovation Team Program (Technology Development Team for High-efficient Silviculture of Forest Resources).
9. CONFLICTS OF INTEREST
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
10. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
11. DATA AVAILABILITY
This study does not involve any huge data acquisition and the corresponding author may be contacted for further assistance with the subject discussed.
12. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Hannah R, Max R. 2013. Land Use. United Kingdom:Our World in Data.
2. Annunzio R, Lindquist E, MacDicken KG. Global Forest Land-use Change from 1990 to 2010:An Update to a Global Remote Sensing Survey of Forests. Rome:Food and Agriculture Organization of the United Nations;2014.
3. Corlett RT, Hughes AC. Mammals in forest ecosystems. In:Routledge Handbook of Forest Ecology. England:Routledge;2015.
4. Baldrian P. Forest microbiome:Diversity, complexity and dynamics. FEMS Microbiol Rev 2017;41:109-30. [CrossRef]
5. Yang W, Min Z, Yang M, Yan J. Exploration of the implementation of carbon neutralization in the field of natural resources under the background of sustainable development-an overview. Int J Environ Res Public Health 2022;19:14109. [CrossRef]
6. Brockerhoff EG, Barbaro L, Castagneyrol B, Forrester DI, Gardiner B, González-Olabarria JR, et al. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers Conserv 2017;26:3005-35. [CrossRef]
7. Debebe B, Senbeta F, Teferi E, Diriba D, Teketay D. Analysis of forest cover change and its drivers in biodiversity hotspot areas of the Semien Mountains National Park, Northwest Ethiopia. Sustainability 2023;15:3001. [CrossRef]
8. Zaki NA, Abd Latif Z. Carbon sinks and tropical forest biomass estimation:A review on role of remote sensing in aboveground-biomass modelling. Geocarto Int 2017;32:701-16. [CrossRef]
9. Asif M, Mughal A, Bisma R, Mehdi Z, Saima S, Ajaz M, et al. Application of different strains of biofertilizers for raising quality forest nursery. Int J Curr Microbiol Appl Sci 2018;7:3680-6. [CrossRef]
10. Ahmad M, Nadeem SM, Naveed M, Zahir ZA. 2016. Potassium-solubilizing bacteria and their application in agriculture. In:Potassium Solubilizing Microorganisms for Sustainable Agriculture. New Delhi:Springer;p.293-313. [CrossRef]
11. Gerten D, Heck V, Jägermeyr J, Bodirsky BL, Fetzer I, Jalava M, et al. Feeding ten billion people is possible within four terrestrial planetary boundaries. Nat Sustain 2020;3:200-8. [CrossRef]
12. Guo J, Li C, Xu X, Sun M, Zhang L. Farmland scale and chemical fertilizer use in rural China:New evidence from the perspective of nutrient elements. J Clean Prod 2022;376:134278. [CrossRef]
13. Liliane TN, Charles MS. 2020. Factors affecting yield of crops. In:Agronomy-Climate Change and Food Security. London:Intech Open;9.
14. PirttiläAM, Tabas HM, Baruah N, Koskimäki JJ. Biofertilizers and biocontrol agents for agriculture:How to identify and develop new potent microbial strains and traits. Microorganisms 2021;9:817. [CrossRef]
15. Macik M, Gryta A, Frac M. 2020. Chapter Two Biofertilizers in Agriculture:An Overview On Concepts, Strategies and Effects on Soil Microorganisms. Vol. 162. Netherlands:Elsevier;31-87. [CrossRef]
16. Smith P, Reay D, Smith J. Agricultural methane emissions and the potential formitigation. Philos Trans A Math Phys Eng Sci 2021;379(2210):20200451. [CrossRef]
17. Nikolaisen M, Cornulier T, Hillier J, Smith P, Albanito F, Nayak D. Methane emissions from rice paddies globally:A quantitative statistical review of controlling variables and modelling of emission factors. J Clean Prod 2023;409:137245. [CrossRef]
18. Zhang C, Ju X, Powlson D, Oenema O, Smith P. Nitrogen surplus benchmarks for controlling N pollution in the main cropping systems of China. Environ Sci Technol 2019;53:6678-87. [CrossRef]
19. Liu S, Lin F, Wu S, Ji C, Sun Y, Jin Y, et al. A meta-analysis of fertilizer-induced soil NO and combined NO+N2O emissions. Glob Change Biol 2017;23:2520-32. [CrossRef]
20. Sponsler DB, Grozinger CM, Hitaj C, Rundlöf M, Botías C, Code A, et al. Pesticides and pollinators:A socioecological synthesis. Sci Total Environ 2019;662:1012-27. [CrossRef]
21. Meena RS, Kumar S, Datta R, Lal R, Vijayakumar V, Brtnicky M, et al. Impact of agrochemicals on soil microbiota and management:A review. Land 2020;9:34. [CrossRef]
22. Rashmi I, Karthika K, Roy T, Shinoji K, Kumawat A, Kala S, et al. 2022. Soil Erosion and sediments:A source of contamination and impact on agriculture productivity. In:Agrochemicals in Soil and Environment:Impacts and Remediation. Berlin:Springer;313-45. [CrossRef]
23. Iwai CB, Khaung T, Prasopsuk J, Ravindran B. Environmental risk assessment of floating gardens in Inle Lake, Myanmar. Urban Climate 2022;44:101194. [CrossRef]
24. Babafemi O, Iyiola AO, Ojeleye AE, Adebayo QS. 2022. Advantages and potential threats of agrochemicals on biodiversity conservation. In:Biodiversity in Africa:Potentials, Threats and Conservation. Berlin:Springer;267-92. [CrossRef]
25. Henze M, van Loosdrecht MC, Ekama GA, Brdjanovic D. 2008. Biological Wastewater Treatment. London:IWA Publishing.
26. Tudi M, Ruan HD, Wang L, Lyu J, Sadler R, Connell D, et al. Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health 2021;18:1112. [CrossRef]
27. Godfray HC, Stephens AE, Jepson PD, Jobling S, Johnson AC, Matthiessen P, et al. A restatement of the natural science evidence base on the effects of endocrine disrupting chemicals on wildlife. Proc R Soc B 2019;286:20182416. [CrossRef]
28. Sharma N, Singhvi R. Effects of chemical fertilizers and pesticides on human health and environment:A review. Int J Agric Environ Biotechnol 2017;10:675-80. [CrossRef]
29. Mahanty T, Bhattacharjee S, Goswami M, Bhattacharyya P, Das B, Ghosh A, et al. Biofertilizers:A potential approach for sustainable agriculture development. Environ Sci Pollut Res 2017;24:3315-35. [CrossRef]
30. Granada CE, Passaglia LM, De Souza EM, Sperotto RA. Is phosphate solubilization the forgotten child of plant growth-promoting rhizobacteria?Front Microbiol 2018;9:2054. [CrossRef]
31. Yadav KK, Sarkar S. Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environment Ecol 2019;37:89-93.
32. Liu W, Poobathy R. Biofertilizer utilization in forestry. In:Biofertilizers:Study and Impact. United States:John Wiley;2021. 1-37. [CrossRef]
33. Jacob SM, Paranthaman S. Biofertilizers:An advent for eco-friendly and sustainable agriculture development. Vegetos 2022;35:1-13. [CrossRef]
34. Paul E. Chapter 1-Soil Microbiology, ecology, and biochemistry:An exciting present and great future built on basic knowledge and unifying concepts. In:Paul, EA editor. Soil Microbiology, Ecology and Biochemistry. 4th ed. Netherlands:Elsevier;2015:1-14. [CrossRef]
35. Bargaz A, Lyamlouli K, Chtouki M, Zeroual Y, Dhiba D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front Microbiol 2018;9:1606. [CrossRef]
36. Singh M, Singh D, Gupta A, Pandey KD, Singh P, Kumar A. Plant growth promoting rhizobacteria:Application in biofertilizers and biocontrol of phytopathogens. PGPR Amelioration in Sustainable Agriculture. Netherlands:Elsevier;2019. 41-66. [CrossRef]
37. Souza PMS, CorroquéNA, Morales AR, Marin-Morales MA, Mei LHI. PLA and organoclays nanocomposites:degradation process and evaluation of ecotoxicity using Allium cepa as test organism. J Polym Environ 2013;21:1052-63. [CrossRef]
38. Lopes MJ, Dias-Filho MB, Gurgel ES. Successful plant growth-promoting microbes:Inoculation methods and abiotic factors. Front Sustain Food Syst 2021;5:606454. [CrossRef]
39. Riaz U, Mehdi SM, Iqbal S, Khalid HI, Qadir AA, Anum W, et al. Bio-fertilizers:eco-friendly approach for plant and soil environment. In:Bioremediation and Biotechnology:Sustainable Approaches to Pollution Degradation. Berlin:Springer;2020. 189-213. [CrossRef]
40. Sneha S, Anitha B, Sahair RA, Raghu N, Gopenath T, Chandrashekrappa G, et al. Biofertilizer for crop production and soil fertility. Acad J Agric Res 2018;6:299-306.
41. Azmat A, Yasmin H, Hassan MN, Nosheen A, Naz R, Sajjad M, et al. Co-application of bio-fertilizer and salicylic acid improves growth, photosynthetic pigments and stress tolerance in wheat under drought stress. PeerJ 2020;8:e9960. [CrossRef]
42. Sunita K, Mishra I, Mishra J, Prakash J, Arora NK. Secondary metabolites from halotolerant plant growth promoting rhizobacteria for ameliorating salinity stress in plants. Front Microbiol 2020;11:567768. [CrossRef]
43. Fasusi OA, Cruz C, Babalola OO. Agricultural sustainability:Microbial biofertilizers in rhizosphere management. Agriculture 2021;11:163. [CrossRef]
44. Lee S, Yap M, Behringer G, Hung R, Bennett JW. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol Biotechnol 2016;3:1-14. [CrossRef]
45. Bumandalai O, Tserennadmid R. Effect of Chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds. Int J Aquat Biol 2019;7:95-9.
46. Mishra J, Prakash J, Arora NK. Role of beneficial soil microbes in sustainable agriculture and environmental management. Climate Change Environ Sustain 2016;4:137-49. [CrossRef]
47. Timmusk S, Behers L, Muthoni J, Muraya A, Aronsson AC. Perspectives and challenges of microbial application for crop improvement. Front Plant Sci 2017;8:49. [CrossRef]
48. Debnath S, Rawat D, Mukherjee AK, Adhikary S, Kundu R. Applications and constraints of plant beneficial microorganisms in agriculture. Biostimulants in Plant Science. London:IntechOpen;2019. [CrossRef]
49. Stamenkovi?S, Beškoski V, Karabegovi?I, Lazi?M, Nikoli?N. Microbial fertilizers:A comprehensive review of current findings and future perspectives. Span J Agric Res 2018;16:e09R01. [CrossRef]
50. Qiu Z, Egidi E, Liu H, Kaur S, Singh BK. New frontiers in agriculture productivity:Optimised microbial inoculants and in situ microbiome engineering. Biotechnol Adv 2019;37:107371. [CrossRef]
51. Calvo P, Nelson L, Kloepper JW. Agricultural uses of plant biostimulants. Plant Soil 2014;383:3-41. [CrossRef]
52. Barragán-Ocaña A, Del Carmen del-Valle-Rivera M. Rural development and environmental protection through the use of biofertilizers in agriculture:An alternative for underdeveloped countries?Technol Soc 2016;46:90-9. [CrossRef]
53. Hazra G. Different types of eco-friendly fertilizers:An overview. Sustain Environ 2016;1:54. [CrossRef]
54. Arora S, Murmu G, Mukherjee K, Saha S, Maity D. A comprehensive overview of nanotechnology in sustainable agriculture. J Biotechnol 2022;355:21-41. [CrossRef]
55. Kumar R, Kumar R, Prakash O. Chapter-5 the impact of chemical fertilizers on our environment and ecosystem. In:Research Trends in Environmental Sciences. Vol. 35. Rohini, New Delhi, India:AkiNik Publications;2019. 69.
56. Talema A. Causes, negative effects, and preventive methods of water pollution in Ethiopia. Qual Assur Saf Crops Foods 2023;15:129-39. [CrossRef]
57. Shamansky S, Pavlyukh L, Gorbachova O, Repeta V. Analysis of concentrations of biogenic compounds discharged into water bodies with municipal wastewater. Environmental safety and nature management 2022;44:15-29. [CrossRef]
58. K?l?çO, Boz ?, Ery?lmaz GA. Comparison of conventional and good agricultural practices farms:A socio-economic and technical perspective. J Clean Prod 2020;258:120666. [CrossRef]
59. Costa MH, da Cunha LC, Cox PM, Eliseev AV, Hensen S, Ishii M, et al. Global Carbon and other Biogeochemical Cycles and Feedbacks. Switzerland:IPCC;2021.
60. Heinz-Ulrich N. Methane emission from rice fields:Wetland rice fields may make a major contribution to global warming. BioScience 1993;43:466-73. [CrossRef]
61. Savci S. An agricultural pollutant:Chemical fertilizer. Int J Environ Sci Dev 2012;3:73. [CrossRef]
62. Shoji S, Delgado J, Mosier A, Miura Y. Use of controlled release fertilizers and nitrification inhibitors to increase nitrogen use efficiency and to conserve air andwater quality. Commun Soil Sci Plant Anal 2001;32:1051-70. [CrossRef]
63. Cooper J, Reed EY, Hörtenhuber S, Lindenthal T, Løes AK, Mäder P, et al. Phosphorus availability on many organically managed farms in Europe. Nutr Cycl Agroecosyst 2018;110:227-39. [CrossRef]
64. Rütting T, Aronsson H, Delin S. Efficient Use of Nitrogen in Agriculture. Berlin:Springer;2018. 1-5. [CrossRef]
65. Sonmez I, Kaplan M, Sonmez S. Investigation of seasonal changes in nitrate contents of soils and irrigation waters in greenhouses Located in Antalya-Demn region. Asian J Chem 2007;19:5639.
66. Sterner RW, Elser JJ. Ecological Stoichiometry. United States:Princeton University Press;2017.
67. Meena VS, Maurya BR, Verma JP, Aeron A, Kumar A, Kim K, et al. Potassium solubilizing rhizobacteria (KSR):Isolation, identification, and K-release dynamics from waste mica. Ecol Eng 2015;81:340-7. [CrossRef]
68. Pindi P. Liquid microbial consortium-a potential tool for sustainable soil health. J Biofertil Biopestic 2012;3:124. [CrossRef]
69. Borkar SG. Microbes as Bio-fertilizers and Their Production Technology. India:Woodhead Publishing Pvt, Ltd;2015. [CrossRef]
70. Verma M, Mishra J, Arora NK. Plant growth-promoting rhizobacteria:Diversity and applications. In:Environmental Biotechnology:For Sustainable Future. Berlin:Springer;2019. 129-73. [CrossRef]
71. MalusáE, Sas-Paszt L, Ciesielska J. Technologies for beneficial microorganisms inocula used as biofertilizers. ScientificWorldJournal 2012;2012:491206. [CrossRef]
72. Wani SA, Chand S, Ali T. Potential use of Azotobacter chroococcum in crop production:An overview. Curr Agric Res J 2013;1:35-8. [CrossRef]
73. Khan HI. Appraisal of biofertilizers in rice:To supplement inorganic chemical fertilizer. Rice Sci 2018;25:357-62. [CrossRef]
74. Bhardwaj D, Ansari MW, Sahoo RK, Tuteja N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Factor 2014;13:66. [CrossRef]
75. Abd El-Fattah DA, Eweda WE, Zayed MS, Hassanein MK. Effect of carrier materials, sterilization method, and storage temperature on survival and biological activities of Azotobacter chroococcum inoculant. Ann Agric Sci 2013;58:111-8. [CrossRef]
76. Parmar P, Sindhu S. Potassium solubilization by rhizosphere bacteria:Influence of nutritional and environmental conditions. J Microbiol Res 2013;3:25-31.
77. Thomas L, Singh I. Microbial biofertilizers:Types and applications. Biofertilizers for Sustainable Agriculture and Environment. Berlin:Springer;2019. 1-19. [CrossRef]
78. Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion:A review. Ann Microbiol 2010;60:579-98. [CrossRef]
79. Egamberdieva D, Wirth SJ, Alqarawi AA, Abd-Allah EF, Hashem A. Phytohormones and beneficial microbes:Essential components for plants to balance stress and fitness. Front Microbiol 2017;8:2104. [CrossRef]
80. Sharma A. Impact of application of chemical fertilizers and biofertilizers on growth and biomass production of mahua seedlings under nursery stages. Advances in Tree Seed Science and Silviculture. Delhi:Global Vision Press;2015. 315.
81. Aloo BN, Tripathi V, Makumba BA, Mbega ER. Plant growth-promoting rhizobacterial biofertilizers for crop production:The past, present, and future. Front Plant Sci 2022;13:1002448. [CrossRef]
82. Thirugnanasambandan T. Advances and Trends in Nano-Biofertilizers. 2018. Available from:SSRN 3306998 [CrossRef]
83. Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, et al. Plant growth-promoting rhizobacteria:Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 2018;9:1473. [CrossRef]
84. Sessitsch A, Howieson JG, Perret X, Antoun H, Martínez-Romero E. Advances in Rhizobium research. Crit Rev Plant Sci 2010;21:323-78. [CrossRef]
85. Mrkovacki N, Milic V. Use of Azotobacter chroococcum as potentially useful in agricultural application. Ann Microbiol 2001;51:145-58.
86. Cassán F, Coniglio A, López G, Molina R, Nievas S, de Carlan CL, et al. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol Fertil Soil 2020;56:461-79. [CrossRef]
87. Tao GC, Tian SJ, Cai MY, Xie GH. Phosphate-solubilizing and-mineralizing abilities of bacteria isolated from soils. Pedosphere 2008;18:515-23. [CrossRef]
88. Halpern M, Bar-Tal A, Ofek M, Minz D, Muller T, Yermiyahu U. The use of biostimulants for enhancing nutrient uptake. Adv Agron 2015;130:141-74. [CrossRef]
89. Egamberdiyeva D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol 2007;36:184-9. [CrossRef]
90. Luo L, Zhao C, Wang E, Raza A, Yin C. Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture:An overview for its mechanisms. Microbiol Res 2022;259:127016. [CrossRef]
91. Ahmed A, Hasnain S. Auxins as one of the factors of plant growth improvement by plant growth promoting rhizobacteria. Polish J Microbiol 2014;63:261-6. [CrossRef]
92. Stewart WM, Roberts TL. Food security and the role of fertilizer in supporting it. Proc Eng 2012;46:76-82. [CrossRef]
93. Daniel AI, Fadaka AO, Gokul A, Bakare OO, Aina O, Fisher S, et al. Biofertilizer:The future of food security and food safety. Microorganisms 2022;10:1220. [CrossRef]
94. Babalola OO. Beneficial bacteria of agricultural importance. Biotechnol Lett 2010;32:1559-70. [CrossRef]
95. Guo J, Muhammad H, Lv X, Wei T, Ren X, Jia H, et al. Prospects and applications of plant growth promoting rhizobacteria to mitigate soil metal contamination:A review. Chemosphere 2020;246:125823. [CrossRef]
96. Mahanty T, Bhattacharjee S, Goswami M, Bhattacharyya P, Das B, Ghosh A, et al. Biofertilizers:A potential approach for sustainable agriculture development. Environ Sci Pollut Res Int 2017;24:3315-35. [CrossRef]
97. Vosátka M, AlbrechtováJ, Patten R. The international market development for mycorrhizal technology. In:Mycorrhiza. Berlin:Springer;2008. 419-38. [CrossRef]
98. Maharana R, Dobriyal MJ, Behera L, Sukhadiya M. Enhancement of seedling vigour through biofertilizers application in gamhar (Gmelinaarborea roxb.). Int J Chem Stud 2018;6:54-60.
99. Raimi A, Roopnarain A, Adeleke R. Biofertilizer production in Africa:Current status, factors impeding adoption and strategies for success. Sci Afr 2021;11:e00694. [CrossRef]
100. Malusa E, Pinzari F, Canfora L. Efficacy of biofertilizers:Challenges to improve crop production. In:Microbial Inoculants in Sustainable Agricultural Productivity. New Delhi:Springer;2016. 17-40. [CrossRef]
101. Masso C, Ochieng JRA, Vanlauwe B. Worldwide contrast in application of bio-fertilizers for sustainable agriculture:Lessons for sub-Saharan Africa. J Biol Agric Healthc 2015;5:34-50.
102. Adeleke RA, Nunthkumar B, Roopnarain A, Obi L. Applications of plant-microbe interactions in agro-ecosystems. In:Microbiome in Plant Health and Disease. Singapore:Springer;2019. 1-34. [CrossRef]
103. Raimi A, Adeleke R, Roopnarain A. Soil fertility challenges and biofertiliser as a viable alternative for increasing smallholder farmer crop productivity in sub-Saharan Africa. Cogent Food Agric 2017;3:1400933. [CrossRef]
104. FiBL. Global Biofertilizers Market;2022. Retractive from https://www.mordorintelligence.com/industry-reports/global-biofertilizers-market-industry?
105. Rashid A, Mir MR, Hakeem KR. Biofertilizer use for sustainable agricultural production. In:Plant, Soil and Microbes. Berlin:Springer;2016. 163-80. [CrossRef]
106. Asoegwu CR, Awuchi CG, Nelson KC, Orji CG, Ulunma NO, Egbufor UC, et al. A review on the role of biofertilizers in reducing soil pollution and increasing soil nutrients. Hmlyn J Agric 2020;1:34-8.
107. Abbas R, Rasul S, Aslam K, Baber M, Shahid M, Mubeen F, et al. Halotolerant PGPR:A hope for cultivation of saline soils. J King Saud Univ Sci 2019;31:1195-201. [CrossRef]
108. Liu A, Wang W, Zheng X, Chen X, Fu W, Wang G, et al. Improvement of the Cd and Zn phytoremediation efficiency of rice (Oryza sativa) through the inoculation of a metal-resistant PGPR strain. Chemosphere 2022;302:134900. [CrossRef]
109. Hossain A, Ali ME, Maitra S, Bhadra P, Rahman MM, Ali S, et al. The role of soil microorganisms in plant adaptation to abiotic stresses:Current scenario and future perspectives. Plant Perspectives to Global Climate Changes. Netherlands:Elsevier;2022. 233-78. [CrossRef]
110. Mitter EK, Tosi M, Obregón D, Dunfield KE, Germida JJ. Rethinking crop nutrition in times of modern microbiology. Innovative biofertilizer technologies. Front Sustain Food Syst 2021;5:606815. [CrossRef]
111. Kong Z, Hart M, Liu H. Paving the way from the lab to the field:using synthetic microbial consortia to produce high-quality crops. Front Plant Sci 2018;9:1467. [CrossRef]
112. Sharma R. Ectomycorrhizal mushrooms:Their diversity, ecology and practical applications. In:Varma A, Prasad R, Tuteja N, editors. Mycorrhiza-Function, Diversity, State of the Art. Cham:Springer International Publishing;2017. 99-131. [CrossRef]
113. Domínguez-Nunez J, Berrocal-Lobo M, Albanesi A. Ectomycorrhizal fungi:Role as biofertilizers in forestry. In:Biofertilizers for Sustainable Agriculture and Environment. Berlin:Springer;2019. 67-82. [CrossRef]
114. Hanif M, Ashraf Z, Bashir S, Riaz F, Amanat R, Yousaf N, et al. Ectomycorrhizal Fungi as Biofertilizers in Forestry. London:IntechOpen;2023. [CrossRef]
115. Rinaldi AC, Comandini O, Kuyper TW. Ectomycorrhizal fungal diversity:Separating the wheat from the chaff. Fungal Divers 2008;33:1-45.
116. Wiensczyk AM, Gamiet S, Durall DM, Jones MD, Simard SW. Ectomycorrhizae and forestry in British Columbia:A summary of current research and conservation strategies. J Ecosyst Manage 2002;2:1-20. [CrossRef]
117. Policelli N, Horton TR, Hudon AT, Patterson TR, Bhatnagar JM. Back to roots:The role of ectomycorrhizal fungi in boreal and temperate forest restoration. Front Forest Global Change 2020;3:97. [CrossRef]
118. Onwuchekwa NE, Zwiazek JJ, Quoreshi A, Khasa DP. Growth of mycorrhizal jack pine (Pinus banksiana) and white spruce (Picea glauca) seedlings planted in oil sands reclaimed areas. Mycorrhiza 2014;24:431-41. [CrossRef]
119. Krumins JA, Goodey NM, Gallagher F. Plant-soil interactions in metal contaminated soils. Soil Biol Biochem 2015;80:224-31. [CrossRef]
120. Luo ZB, Wu C, Zhang C, Li H, Lipka U, Polle A. The role of ectomycorrhizas in heavy metal stress tolerance of host plants. Environ Exp Bot 2014;108:47-62. [CrossRef]
121. Jourand P, Hannibal L, Majorel C, Mengant S, Ducousso M, Lebrun M. Ectomycorrhizal pisolithus albus inoculation of Acacia spirorbis and Eucalyptus globulus grown in ultramafic topsoil enhances plant growth and mineral nutrition while limits metal uptake. J Plant Physiol 2014;171:164-72. [CrossRef]
122. Sterkenburg E, Clemmensen KE, Lindahl BD, Dahlberg A. The significance of retention trees for survival of ectomycorrhizal fungi in clear-cut Scots pine forests. J Appl Ecol 2019;56:1367-78. [CrossRef]
123. Glassman SI, Levine CR, DiRocco AM, Battles JJ, Bruns TD. Ectomycorrhizal fungal spore bank recovery after a severe forest fire:Some like it hot. ISME J 2016;10:1228-39. [CrossRef]
124. Dove NC, Hart SC. Fire reduces fungal species richness and in situ mycorrhizal colonization:A meta-analysis. Fire Ecol 2017;13:37-65. [CrossRef]
125. Patterson TR. Legacy of Robinia pseudoacacia Invasion and Use of Ectomycorrhizal fungi to Restore Pinus rigida. Albany Pine Bush Preserve, NY:State University of New York College of Environmental Science and Forestry;2019.
126. Nuñez MA, Chiuffo MC, Torres A, Paul T, Dimarco RD, Raal P, et al. Ecology and management of invasive Pinaceae around the world:Progress and challenges. Biol Invas 2017;19:3099-120. [CrossRef]
127. Policelli N, Bruns TD, Vilgalys R, Nuñez MA. Suilloid fungi as global drivers of pine invasions. New Phytol 2019;222:714-25. [CrossRef]
128. Dickie IA, Nuñez MA, Pringle A, Lebel T, Tourtellot SG, Johnston PR. Towards management of invasive ectomycorrhizal fungi. Biol Invas 2016;18:3383-95. [CrossRef]
129. Lyu D, Smith DL. The root signals in rhizospheric inter-organismal communications. Front Plant Sci 2022;13:1064058. [CrossRef]
130. Aioub AA, Elesawy AE, Ammar EE. Plant growth promoting rhizobacteria (PGPR) and their role in plant-parasitic nematodes control:A fresh look at an old issue. J Plant Dis Prot 2022;129:1305-21. [CrossRef]
131. Heydari M, Hajinia S, Jafarian N, Karamian M, Mosa Z, Asgharzadeh S, et al. Synergistic use of biochar and the plant growth-promoting rhizobacteria in mitigating drought stress on oak (Quercus brantii Lindl.) seedlings. Forest Ecol Manag 2023;531:120793. [CrossRef]
132. Shi JW, Lu LX, Shi HM, Ye JR. Effects of plant growth-promoting rhizobacteria on the growth and soil microbial community of Carya illinoinensis. Curr Microbiol 2022;79:352. [CrossRef]
133. Kong W, Xu X, Li Z, Wang Y, Wu X. Combining ectomycorrhizal fungi and plant growth-promoting rhizobacteria to enhance salt tolerance of Metasequoia glyptostroboides. J Forest Res 2022;84:195-208. [CrossRef]
134. Renaudin M, Blasi C, Bradley RL, Bellenger JP. New insights into the drivers of moss-associated nitrogen fixation and cyanobacterial biomass in the eastern Canadian boreal forest. J Ecol 2022;110:1403-18. [CrossRef]
135. Kubota M, Matsushita N, Nakamura T, Fukuda K. Nitrogen fixation and nifH gene diversity in Cyanobacteria living on feather mosses in a subalpine forest of Mt. Fuji. Oecologia 2023;201:749-60. [CrossRef]
136. Ennab H. Effect of organic manures, biofertilizers and NPK on vegetative growth, yield, fruit quality and soil fertility of eureka lemon trees (Citrus limon (L.) Burm). J Soil Sci Agric Eng 2016;7:767-74. [CrossRef]
137. Loong C. Exploring Growth Enhancing Rhizospheric Microorganisms for Silviculture of Eucalyptus pellita;2018.
138. Nasir J, Ashfaq M, Baig I, Khair SM, Mangan T, Allan C, et al. Representative agricultural pathways and socioeconomic benefits of groundwater management interventions in Punjab, Sindh and Balochistan Provinces, Pakistan. Albury, NSW:Institute for Land, Water and Society;2021.
139. Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH. Biostimulants in plant science:A global perspective. Front Plant Sci 2017;7:2049. [CrossRef]
140. Herrmann L, Lesueur D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl Microbiol Biotechnol 2013;97:8859-73. [CrossRef]
141. Namasivayam SK, Saikia SL, Bharani A. Evaluation of persistence and plant growth promoting effect of bioencapsulated formulation of suitable bacterial bio-fertilizers. Biosci Biotech Res Asia 2014;11:407-15. [CrossRef]
142. Nehra V, Choudhary M. A review on plant growth promoting rhizobacteria acting as bioinoculants and their biological approach towards the production of sustainable agriculture. J Appl Nat Sci 2015;7:540-56. [CrossRef]
143. Bashan Y, de-Bashan LE, Prabhu S, Hernandez JP. Advances in plant growth-promoting bacterial inoculant technology:Formulations and practical perspectives (1998-2013). Plant Soil 2014;378:1-33. [CrossRef]
144. Avishek D, Hayat U, Ferdous Z. Utilization of by-products from food processing as biofertilizers and biopesticides. In:Food Processing By-Products and their Utilization. United States:Wiley;2017. 175-93. [CrossRef]
145. Brahmaprakash G, Sahu PK, Lavanya G, Nair SS, Gangaraddi VK, Gupta A. Microbial functions of the rhizosphere. In:Plant-microbe Interactions in Agro-Ecological Perspectives. Berlin:Springer;2017. 177-210. [CrossRef]
146. Kaminsky LM, Trexler RV, Malik RJ, Hockett KL, Bell TH. The inherent conflicts in developing soil microbial inoculants. Trends Biotechnol 2019;37:140-51. [CrossRef]
147. Vassilev N, Vassileva M, Martos V, Garcia del Moral LF, Kowalska J, Tylkowski B, et al. Formulation of microbial inoculants by encapsulation in natural polysaccharides:Focus on beneficial properties of carrier additives and derivatives. Front Plant Sci 2020;11:270. [CrossRef]
148. Berninger T, González López Ó, Bejarano A, Preininger C, Sessitsch A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb Biotechnol 2018;11:277-301. [CrossRef]
149. Vassilev N, Mendes G, Costa M, Vassileva M. Biotechnological tools for enhancing microbial solubilization of insoluble inorganic phosphates. Geomicrobiol J 2014;31:751-63. [CrossRef]
150. Bashan Y, de-Bashan LE, Prabhu S. Superior polymeric formulations and emerging innovative products of bacterial inoculants for sustainable agriculture and the environment. In:Agriculturally Important Microorganisms. Singapore:Springer;2016. 15-46. [CrossRef]
151. John RP, Tyagi R, Brar S, Surampalli R, Prévost D. Bio-encapsulation of microbial cells for targeted agricultural delivery. Crit Rev Biotechnol 2011;31:211-26. [CrossRef]
152. Sahu P, Brahmaprakash G. Formulations of biofertilizers-approaches and advances. In:Microbial Inoculants in Sustainable Agricultural Productivity. New Delhi:Springer;2016. 179-98. [CrossRef]
153. Sahu P, Lavanya G, Brahmaprakash G. Fluid bed dried microbial inoculants formulation with improved survival and reduced contamination level. J Soil Biol Ecol 2013;33:81-94.
154. Arora NK, Mishra J. Prospecting the roles of metabolites and additives in future bioformulations for sustainable agriculture. Appl Soil Ecol 2016;107:405-7. [CrossRef]
155. Kamilova F, Okon Y, de Weert S, Hora K. Commercialization of microbes:Manufacturing, inoculation, best practice for objective field testing, and registration. Principles of Plant-microbe Interactions. Cham:Springer;2015. 319-27. [CrossRef]
156. Hayat W, Aman H, Irshad U, Azeem M, Iqbal A, Nazir R. Analysis of ecological attributes of bacterial phosphorus solubilizers, native to pine forests of Lower Himalaya. Appl Soil Ecol 2017;112:51-9. [CrossRef]
157. Basu A, Prasad P, Das SN, Kalam S, Sayyed R, Reddy M, et al. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants:Recent developments, constraints, and prospects. Sustainability 2021;13:1140. [CrossRef]
158. Ji SH, Kim JS, Lee CH, Seo HS, Chun SC, Oh J, et al. Enhancement of vitality and activity of a plant growth-promoting bacteria (PGPB) by atmospheric pressure non-thermal plasma. Sci Rep 2019;9:1044. [CrossRef]
159. Krishnaprabu S. Liquid microbial consortium:A potential tool for sustainable soil health. J Pharmacogn Phytochem 2020;9:2191-9. [CrossRef]
160. Kaljeet S, Keyeo F, Amir H. Influence of carrier materials and storage temperature on survivability of rhizobial inoculant. Asian J Plant Sci 2011;10:331. [CrossRef]
161. Choo KH, Deraman M, Rahim KA. Evaluation of storage temperatures to meet shelf life requirement for acinetobacter baumannii biofertilizer product. J Sains Nukl Malay 2022;34:9-14.
162. Phiromtan M, Mala T, Srinives P. Effect of various carriers and storage temperatures on survival of Azotobacter vinelandii NDD-CK-1 in powder inoculant. Modern Appl Sci 2013;7:81. [CrossRef]
163. Roy A. Biofertilizers for agricultural sustainability:Current status and future challenges. In:Current Trends in Microbial Biotechnology for Sustainable Agriculture. Singapore:Springer;2021. 525-53. [CrossRef]
164. Lawal TE, Babalola OO. Relevance of biofertilizers to agriculture. J Hum Ecol 2014;47:35-43. [CrossRef]
165. Zambrano-Mendoza JL, Sangoquiza-Caiza CA, Campaña-Cruz DF, Yánez-Guzmán CF. Use of biofertilizers in agricultural production. In:Technology in Agriculture. London:Intech Open;2021. 193. [CrossRef]
166. Naveed M, Mehboob I, Shaker MA, Hussain MB, Farooq M. Biofertilizers in Pakistan:Initiatives and limitations. Int J Agric Biol 2015;17:411-20. [CrossRef]
167. Owen D, Williams A, Griffith G, Withers P. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl Soil Ecol 2015;86:41-54. [CrossRef]
168. Behera B, Paramasivan B. Research trends and market opportunities of microalgal biorefinery technologies from circular bioeconomy perspectives. Bioresour Technol 2022;351:127038. [CrossRef]
169. FAQs. Biofertilizers Global Market Report 2023-by Product (Nitrogen Fixing, Phosphate Solubilizing, Other Products), By Crop Type (Cereals and Grains, Oilseeds and Pulses, Fruits and Vegetables, Other Crop Types), By Form (Liquid, Carrier based), By Application (Seed Treatment, Soil Treatment)-Market Size, Trends, And Global Forecast 2023-2032;2023.
170. Saritha M, Tollamadugu NP. The status of research and application of biofertilizers and biopesticides:Global scenario. In:Recent Developments in Applied Microbiology and Biochemistry. Netherlands:Elsevier;2019. 195-207. [CrossRef]
171. Kowalewski O, ?piewanowski P. Stock market response to potash mine disasters. J Commod Mark 2020;20:100124. [CrossRef]
172. Joshi SK, Gauraha AK. Global biofertilizer market:Emerging trends and opportunities. In:Trends of Applied Microbiology for Sustainable Economy. Netherlands:Elsevier;2022. 689-97. [CrossRef]
173. Riaz U, Murtaza G, Anum W, Samreen T, Sarfraz M, Nazir MZ. Plant growth-promoting rhizobacteria (PGPR) as biofertilizers and biopesticides. In:Microbiota and Biofertilizers. Cham:Springer;2021. 181-96. [CrossRef]
174. Gamalero E, Bona E, Glick BR. Current techniques to study beneficial plant-microbe interactions. Microorganisms 2022;10:1380. [CrossRef]
175. Jha P, Kumar V. Role of metagenomics in deciphering the microbial communities associated with rhizosphere of economically important plants. In:Current Trends in Microbial Biotechnology for Sustainable Agriculture. Singapore:Springer;2021. 79-94. [CrossRef]
176. Saad MM, Eida AA, Hirt H. Tailoring plant-associated microbial inoculants in agriculture:A roadmap for successful application. J Exp Bot 2020;71:3878-901. [CrossRef]
177. Toju H, Peay KG, Yamamichi M, Narisawa K, Hiruma K, Naito K, et al. Core microbiomes for sustainable agroecosystems. Nat Plants 2018;4:247-57. [CrossRef]
178. Diwan D, Rashid M, Vaishnav A. Current understanding of plant-microbe interaction through the lenses of multi-omics approaches and their benefits in sustainable agriculture. Microbiol Res 2022;265:127180. [CrossRef]
179. Vidal C, González F, Santander C, Pérez R, Gallardo V, Santos C, et al. Management of rhizosphere microbiota and plant production under drought stress:A comprehensive review. Plants 2022;11:2437. [CrossRef]
180. Rai S, Omar AF, Rehan M, Al-Turki A, Sagar A, Ilyas N, et al. Crop microbiome:Their role and advances in molecular and omic techniques for the sustenance of agriculture. Planta 2022;257:27. [CrossRef]
181. Adeleke BS, Babalola OO. Meta-omics of endophytic microbes in agricultural biotechnology. Biocatal Agric Biotechnol 2022;42:102332. [CrossRef]
182. Yang G, Yang Y, Zang H, Zhao B, Hu Y, Gao Q, et al. Effects of biofertilizer substituting synthetic nitrogen fertilizer on growth and yield of naked oat in semi-arid area. J Inner Mongolia Agric Univ 2022;43.
183. Liu Y, Li G, Zhang H, Wang Q, Chen J, Suo M. Effect of urea and biofertilizer on the growth and yield of Pennisetum giganteum Z. X. Lin. J Inner Mongolia Agric Univ 2022;43:1009-3575.
184. Jia Y, Liao Z, Wang LF, Bu JC, Lin BS, Lin H, et al. Effects of chemical fertilizer reduction and co-application with a JUNCAO nitrogen-fixing biofertilizer on growth and nutritional quality of Pennisetum giganteum and soil nutrient status. Acta Prataculturae Sin 2021;30:215-23.
185. Zhang Y. Research on Farmers'Response Mechanism of Biological Fertilizer Technology Compensation in Cotton Fields in Xinjiang[D]. Shihezi:Shihezi University;2021.
186. Verma P, Yadav AN, Kumar V, Singh DP, Saxena AK. Beneficial plant-microbes interactions:Biodiversity of microbes from diverse extreme environments and its impact for crop improvement. In:Plant-microbe Interactions in Agro-Ecological Perspectives. Berlin:Springer;2017. 543-80. [CrossRef]
187. Valverde A, Burgos A, Fiscella T, Rivas R, Velazquez E, Rodríguez-Barrueco C, et al., editors. Differential effects of coinoculations with Pseudomonas jessenii PS06 (a phosphate-solubilizing bacterium) and Mesorhizobium ciceri C-2/2 strains on the growth and seed yield of chickpea under greenhouse and field conditions. In:First International Meeting on Microbial Phosphate Solubilization. Berlin:Springer;2007.
188. Clayton G, Rice W, Lupwayi N, Johnston A, Lafond G, Grant C, et al. Inoculant formulation and fertilizer nitrogen effects on field pea:Nodulation, N2 fixation and nitrogen partitioning. Can J Plant Sci 2004;84:79-88. [CrossRef]
189. Shoeip AM, Hameed AE, Samia M, Gad DA, Aboud FS. Improving growth, yield and essential oil of lavander (Lavandula officinalis) L. by using compost and biofertilizer application in clay soil. Egypt J Agric Res 2022;100:357-70.
190. Puri A, Padda KP, Chanway CP. Can naturally-occurring endophytic nitrogen-fixing bacteria of hybrid white spruce sustain boreal forest tree growth on extremely nutrient-poor soils?Soil Biol Biochem 2020;140:107642. [CrossRef]
191. Ahsan ML, Ali A, Ahmed I. Biofertiliser a highly potent alternative to chemical fertilisers:Uses and future prospects. J Chem Eng Biol Sci 2012;6:10-23.
192. Upadhyay SK, Singh JS, Saxena AK, Singh DP. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol 2012;14:605-11. [CrossRef]
193. Mohamed AH, Abd El-Megeed FH, Hassanein NM, Youseif SH, Farag PF, Saleh SA, et al. Native Rhizospheric and Endophytic fungi as sustainable sources of plant growth promoting traits to improve wheat growth under low nitrogen input. J Fungi (Basel) 2022;8:94. [CrossRef]
194. Teymouri M, Akhtari J, Karkhane M, Marzban A. Assessment of phosphate solubilization activity of Rhizobacteria in mangrove forest. Biocatal Agric Biotechnol 2016;5:168-72. [CrossRef]
195. Wei Y, Zhao Y, Shi M, Cao Z, Lu Q, Yang T, et al. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour Technol 2018;247:190-9. [CrossRef]
196. Etesami H, Emami S, Alikhani HA. Potassium solubilizing bacteria (KSB):Mechanisms, promotion of plant growth, and future prospects A review. J Soil Sci Plant Nutr 2017;17:897-911. [CrossRef]
197. Jha Y. Potassium mobilizing bacteria:Enhance potassium intake in paddy to regulates membrane permeability and accumulate carbohydrates under salinity stress. Braz J Biol Sci 2017;4:333-44. [CrossRef]
198. Gandhi R, Prittesh P, Jinal HN, Chavan SM, Paul D, Amaresan N. Evaluation of the effect of potassium solubilizing bacterial strains on the growth of wheat (Triticum aestivum L.). J Plant Nutr 2022;46:1-12. [CrossRef]
199. Naeem U, Afzaal M, Qazi A, Yasar A, Mahfooz Y, Naz AU, et al. Investigating the effect of Aspergillus niger inoculated press mud (biofertilizer) on the potential of enhancing maize (Zea mays. L) yield, potassium use efficiency and potassium agronomic efficiency. Cereal Res Commun 2022;50:157-70. [CrossRef]
200. Mohamed AA, Eweda WE, Heggo A, Hassan EA. Effect of dual inoculation with arbuscular mycorrhizal fungi and sulphur-oxidising bacteria on onion (Allium cepa L.) and maize (Zea mays L.) grown in sandy soil under green house conditions. Ann Agric Sci 2014;59:109-18. [CrossRef]
201. Najafi S, Nasi HN, Tuncturk R, Tuncturk M, Sayyed RZ, Amirnia R. Biofertilizer application enhances drought stress tolerance and alters the antioxidant enzymes in medicinal pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae 2021;7:588. [CrossRef]
202. Kamran S, Shahid I, Baig DN, Rizwan M, Malik KA, Mehnaz S. Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front Microbiol 2017;8:2593. [CrossRef]
203. Zahra ST, Tariq M, Abdullah M, Azeem F, Ashraf MA. Dominance of Bacillus species in the wheat (Triticum aestivum L.) rhizosphere and their plant growth promoting potential under salt stress conditions. PeerJ 2023;11:e14621. [CrossRef]
204. Mumtaz MZ, Ahmad M, Zafar-ul-Hye M, Saqib M, Akhtar MF, Zaheer MS. Seed-applied zinc-solubilising Bacillus biofertilisers improve antioxidant enzyme activities, crop productivity, and biofortification of maize. Crop Past Sci 2022;73:503-14. [CrossRef]
205. Srivastava R, Govil M. Influence of biofertilizers on growth and flowering in gladiolus CV. American beauty. In:International Conference and Exhibition on Soilless Culture ICESC. Vol. 742. Leuven, Belgium:ISHS;2005. 183-8. [CrossRef]
206. Hoda E, Mona S. Effect of bio and chemical fertilizers on growth and flowering of Petunia hybrida plants. Am J Plant Physiol 2014;9:68-77. [CrossRef]
207. Zulueta-Rodriguez R, Cordoba-Matson MV, Hernandez-Montiel LG, Murillo-Amador B, Rueda-Puente E, Lara L. Effect of Pseudomonas putida on growth and anthocyanin pigment in two poinsettia (Euphorbia pulcherrima) cultivars. ScientificWorld Journal 2014;2014:810192. [CrossRef]
208. Singh R, Pandey D, Kumar A, Singh M. PGPR isolates from the rhizosphere of vegetable crop Momordica charantia:Characterization and application as biofertilizer. Int J Curr Microbiol Appl Sci 2017;6:1789-02. [CrossRef]
209. Modi K, Jha S, Patel P, Suthar H. Isolation and characterization of Bacillus consortia for plant growth promotion in rice (Oryza sativa L.). Plant Sci 2022;5:17-28. [CrossRef]
210. Barriuso J, Solano BR, Santamaria C, Daza A, Mañero FG. Effect of inoculation with putative plant growth-promoting rhizobacteria isolated from Pinus spp. on Pinus pinea growth, mycorrhization and rhizosphere microbial communities. J Appl Microbiol 2008;105:1298-309. [CrossRef]